Rep:Mod:syc2025
Techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
Shuk Yi Chan
The Hydrogenation of Cyclopentadiene Dimer
Part 1
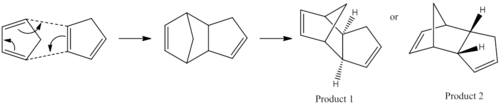
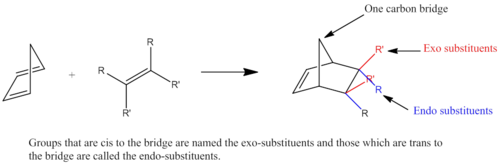
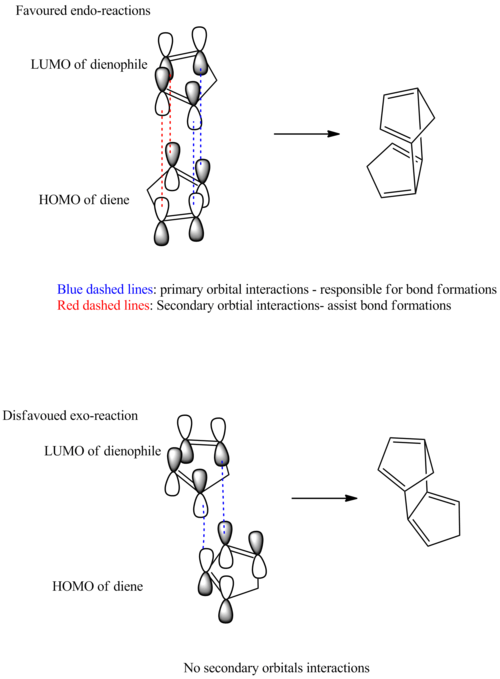
Cyclopentadiene can dimerise through Diel Alder reaction, in which one cyclopentadiene would behave as a diene and the other acts as dienophile. As a result, one of the π systems contributes 4 electrons and the other 2 π electrons, resulting in a thermal reaction proceeding via a Huckel topology. Therefore the system can be classified as a π4s + π2s cycloaddition reaction. If both bonds form on the bottom face of the diene and the top face of the alkene, this is the exo form. Whereas the endo isomer is when the bonds are formed to the bottom face of the alkene.
Therefore depending on the condition of the reaction, the endo or exo product could be favoured. In a thermodynamic reaction, an equilibrium in established within the system and the most stable product is formed. Whereas, the kinetically controlled reaction is more dependent upon the stability of the transition state (which has a lower activation energy that the endothermic reaction) and proceed irreversibly. Therefore, through calculating the energies of the two products it is possible to investigate their energies.
The minimum energy of product 1 and 2 were calculated using ChemBio3D Ultra 12.0 software through MM2 force field. (all the parameters used have been finalized)
| Product 1(kcal/mol) | Product 2 (kcal/mol) | Difference (kcal/mol) | |
|---|---|---|---|
| Stretch | 1.2845 | 1.2519 | 0.0326 |
| Bend | 20.5806 | 20.847 | -0.2664 |
| Stretch-bend | -0.8379 | -0.8359 | -0.002 |
| Torsion | 7.655 | 9.5108 | -1.8558 |
| Non - 1,4 VDW | -1.4175 | -1.5433 | 0.1258 |
| 1,4 VDW | 4.2342 | 4.3195 | -0.0853 |
| Dipole/dipole | 0.3775 | 0.4476 | -0.0701 |
| Total energy | 31.8765 | 33.9975 | -2.121 |
Between the two isomers, there is a total energy difference of approximately -2.12Kcal/mol ; with the exo isomer being the more energetically stable molecule. Therefore the exo-isomer is the thermodynamic product and the endo-isomer is the kinetic one. Therefore the diels alder dimerization reaction was controlled by kinetic constraints. Generally the exo-isomer are favoured due to steric grounds, whilst the endo-isomer is preferred over electronic grounds (secondary orbital interactions). Secondary orbital interactions compacts the diene-dienophile complex, which facilitates the reaction and provide additional electron delocalisation. This leads to a lowering in energy for the transition state leading to a faster rate of reaction.
Part 2
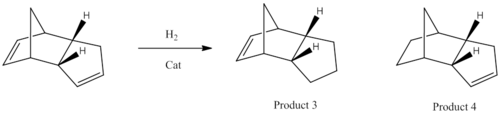
The endo-Cyclopentadiene dimer can be hydrolysed by H2 with a metal catalyst (eg. palladium) to form either product 3 or 4. Their corresponding energies are investigated through the MM2 forcefield.
| Product 3(kcal/mol) | Product 4(kcal/mol) | Difference (kcal/mol) | |
|---|---|---|---|
| Stretch | 1.2771 | 1.0972 | 0.1799 |
| Bend | 19.8664 | 14.5278 | 5.3386 |
| Stretch-bend | -0.8346 | -0.5496 | -0.285 |
| Torsion | 10.8068 | 12.4972 | -1.6904 |
| Non - 1,4 VDW | -1.2257 | -1.0722 | -0.1535 |
| 1,4 VDW | 5.6330 | 4.5110 | 1.122 |
| Dipole/dipole | 0.1621 | 0.1406 | 0.0215 |
| Total energy | 35.6850 | 31.1520 | 4.533 |
Between the two molecule, there is a difference of approximately 4.53Kcal/mol, Product 4 having the lower energy. In terms of stretches, stretch bends, Non-1,44 vdw and dipople/dipole energy; there is only a small changes. This means that they are not greatly affected by the allocation of the double bond within the molecule. Whereas there is a greater contribution from the torsion (larger for product 3) and 1,4 van der waals (larger for product 4) energy, which mean that their energy is effected by the selective hydrogenation of which double bond. Torsion is the energy required to rotate a single bond, which is dependent on the dihedral angle of the bond.
The greatest contribution to the difference in energy is the bend energy – this is the energy required to bend a bond from its equilibrium angle. The energy of this would have been calculated by the Hookian potential; which is dependent on the angle.
Ebend = ½Kb,ijk(Θijk - Θo)2
Where Kb,ijk is the bending force constant and Θijk is the instantaneous bond angle.
Product 3 has a bond angle of 107.6o between the C-C=C bond and product 4 has an angle of 112.8o. For product 4, the ideal bond angle for a 5 member ring (theoretical planar) is 108o; therefore there would only be a low level of bond strain present in the system due to the presence of the double bond. In a general cyclohexane, ring strain and eclipsing interactions are negligible, as the puckering of the ring allows the ideal tetrahedral bond angle to be achieved. The tetrahedral bond corresponds well to the product 3 bond angle; therefore it indicates that this is not the reason why there is an increase in bend energy.
Another argument is that the carbons are sp2 hybridised, which means that their ideal bond angle is 120o. Therefore, product 4 is closer to the ideal bond angle than product 3. Which means that hydrogenation of the double bond in the bicyclic ring would lead to a greater decrease in bond strain and overall energy of the system.
Practical experiments show that product 4 is the main product that is formed; With the hydrogenation of the norbornene being five time faster than the hydrogenation of the cyclopentene ring.[1]
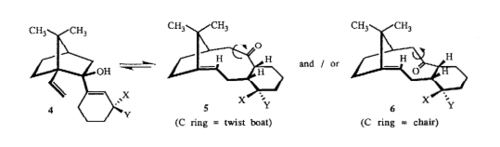
Atropisomers are stereoisomers with restricted rotation around a single bond, in which the steric strain barrier for rotation is sufficiently high enough for the isolation of the two conformer.[3] Product 9 and product 10 is an example of atropisomers, with a hindered rotation adjacent to the C=O bond; resulting in two different conformation - twist boat and chair.
| Product 10(kcal/mol) | Product 9(kcal/mol) | Difference (kcal/mol) | |
|---|---|---|---|
| Stretch | 2.9134 | 3.2569 | -0.3435 |
| Bend | 16.8143 | 19.2510 | -2.4367 |
| Stretch-bend | 0.4403 | 0.3322 | 0.1081 |
| Torsion | 20.2312 | 20.6337 | -0.4025 |
| Non - 1,4 VDW | 0.1351 | 0.7654 | -0.6303 |
| 1,4 VDW | 14.3528 | 15.3734 | -1.0206 |
| Dipole/dipole | -1.7228 | -1.8300 | 0.10719 |
| Total energy | 53.1644 | 57.7827 | -4.6183 |
There is a small energy difference of approximately 4.6183kcal/mol between the two conformation. With the main contributor to this being the bend energy. The chair conformation in product 10 is significantly more flexible than the twist boat; therefore less energy is required for it to bend.
For product 10
MFF94 Minimization
Iteration 43: Minimization terminated normally because the gradient norm is less than the minimum gradient norm
Final Energy: 77.8972 kcal/mol
Calculation completed
MMFF94 Minimization
Iteration 78: Minimization terminated normally because the gradient norm is less than the minimum gradient norm
Final Energy: 82.7741 kcal/mol
Calculation completed
Hyperstable alkenes focus on the stability of the bridgehead alkenes (such as the norbornene rings) - it is believed that these olefins are considerably more stable than normal alkene. As they have less strain than the corresponding hydrocarbon; they are less reactive.
Modelling Using Semi-empirical Molecular Orbital Theory.
| MM2(kcal/mol) | MOPAC(PM6)(kcal/mol) | MOPAC(RM1)(kcal/mol) | |
|---|---|---|---|
| Stretch | 0.6187 | ||
| Bend | 4.7238 | ||
| Stretch-bend | 0.0397 | ||
| Torsion | 7.6660 | ||
| Non - 1,4 VDW | -1.0620 | ||
| 1,4 VDW | 5.7959 | ||
| Dipole/dipole | 0.1124 | ||
| Total energy | 17.8946 | 19.74006 | 22.82759 |
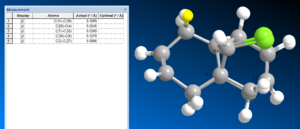
Pentahelicene |
The two optimized molecule (MM2 and MopacPM6) are overlapped together in order to compare the difference in structure. The overlap was relatively successful which indicate that the structures are very similar with only a small difference. That had led to the 0.1Å distance between some of the atoms. MOPAC appear to exhibit a greater distortion away from the chlorine ring than mm2.
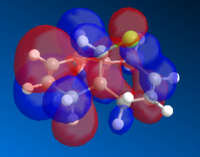
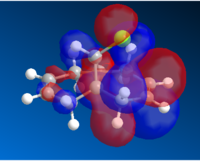
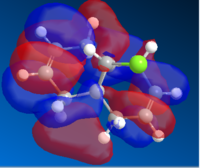
The Molecular orbitals for the systems are:
| HOMO-1 | HOMO | LUMO | LUMO+1 | LUMO+2 |
|---|---|---|---|---|
 |
 |
 |
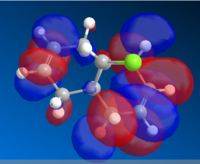 |
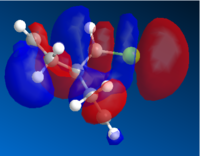
|
The orbitals shows that there is a greater level of bonding on the side with the chlorine bond; as in the HOMO the electron density are centered around that specific ring. As it is more electron rich and nucleophilic; there will be a greater possibility that the addition of carbene would be regioselective to the endo alkene. Also, the orbitals are relatively large and diffuse, which would allow thee carbene to attack more readily.
Whereas the LUMO indicate that there is a high level of anti-bonding in the alkene ring opposite to the chlorine bond. Which means that it would be unlikely that a bond at that position would be unfavorable.
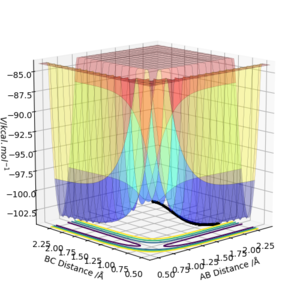
The molecular electrostatic potnetial consider the total electron distribution, rather than a single molecular orbital.The MEP has both positive surfaces repulsive (to H+ as an electrophile) and attractive to an electrophile. Positive electrostatic potential corresponds to repulsion of the proton by the atomic nuclei in regions where low electron density exists and the nuclear charge is incompletely shielded(colored in shades of blue). Negative electrostatic potential corresponds to a attraction of the proton by the concentrated electron density in the molecules (from lone pairs, pi-bonds, etc.) (colored in shades of red).
.
The molecular vibration was calculated:
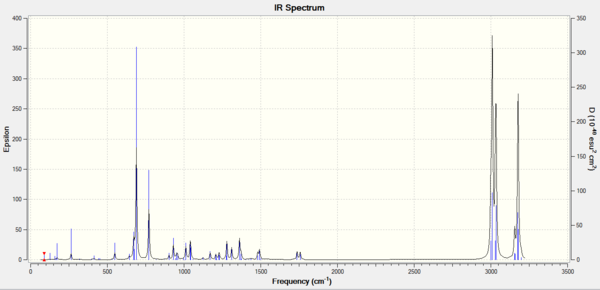
Monosaccharide chemistry and the mechanism of glycosidation
Glycosidation involves replacing the group X by reaction with a nucleophile Nu as shown on the right. This reaction is highly stereospecific. The stereochemistry of the C-OAc bond (shown in red) controls the stereochemical outcome of the C-Nu bond (shown in green) by nucleophilic attack at the carbon shown in blue. This effect is due to neighbouring-group-participation from the adjacent acetyl group in the intermediate A to form a second intermediate oxenium cation B, which is then itself displaced by an incoming nucleophile attacking from either the top face to form the β-anomer or the bottom face to form the α-anomer of the final product.
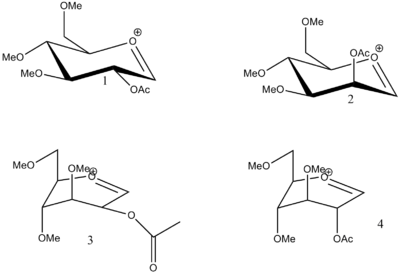
| Product 1(kcal/mol) | Product 2(kcal/mol) | Product 3(kcal/mol) | Product 4(kcal/mol) | |
|---|---|---|---|---|
| Stretch | 2.1611 | 2.5124 | 2.3423 | 2.2061 |
| Bend | 13.0514 | 14.2542 | 8.9865 | 13.7278 |
| Stretch-bend | 0.9318 | 0.9969 | 0.8063 | 0.9556 |
| Torsion | 2.0221 | 0.0223 | 1.6196 | 0.6128 |
| Non - 1,4 VDW | -3.0790 | -1.7670 | -2.5983 | -1.7635 |
| 1,4 VDW | 19.1999 | 18.0837 | 19.3700 | 18.7351 |
| Charge/dipole | -4.0566 | -5.4986 | -4.1021 | -4.3401 |
| Dipole/dipole | 5.4556 | 7.6760 | 3.9255 | 8.6190 |
| Total energy | 35.6874 | 36.2799 | 30.3498 | 28.7527 |
| MOPAC | -75.90739 | -68.23414 | -89.24354 | -62.91862 |
The bond angles and bond lengths of the molecule could be compared:
| mode# | Product 1 | Product 2 | Product 3 | Product 4 |
|---|---|---|---|---|
| Bond length (nm) | 0.433 | 0.434 | 0.238 | 0.436 |
| Bond angle | 133.2 | 164.9 | 104.4 | 102.8 |
Produt 3 and 4 both have a bond angle that is close to the Burgi-Dunitz angle; which may suggest that nucleophilic attack would be more favorable. The long bond distance may be an indication that the optimization failed to find its minimum energy structure, as the orientation of the c=o bond seems to be positioned awkwardly in the molecules.
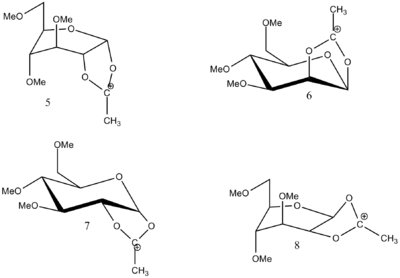
| Product 5(kcal/mol) | Product 6(kcal/mol) | Product 7(kcal/mol) | Product 8(kcal/mol) | |
|---|---|---|---|---|
| Stretch | 2.0996 | 2.6058 | 2.6562 | 2.6369 |
| Bend | 12.9149 | 16.0969 | 14.8859 | 14.3962 |
| Stretch-bend | 0.7604 | 0.9170 | 0.9316 | 0.9296 |
| Torsion | 5.7627 | 10.2772 | 8.8427 | 8.8369 |
| Non - 1,4 VDW | -5.1114 | -1.6775 | -3.0253 | -3.0165 |
| 1,4 VDW | 20.2034 | 22.5763 | 21.3522 | 21.2082 |
| Charge/dipole | 1.8282 | -21.7843 | -3.6158 | -3.6158 |
| Dipole/dipole | 9.5652 | 9.2318 | 9.9060 | 9.9060 |
| Total energy | 48.0232 | 38.2433 | 51.2815 | 51.2815 |
| MOPAC | -91.66305 | -88.40996 | -66.87728 | -66.88190 |
(literature reference)[4]
Week 2
| Stretch | 3.4031 |
| Bend | 12.1224 |
| Stretch-bend | 0.3536 |
| Torsion | 20.9816 |
| Non - 1,4 VDW | -2.8279 |
| 1,4 VDW | 15.2813 |
| Dipole/dipole | -2.8614 |
| Total energy | 46.4526 kcal/mol |
The molecule is then minimized using the DFT=B3LYP method, with the basis set of 6-31G + d (Polarisation) + p(H polarisation); and the solvent used was chloroform.DOI:10042/23316 The NMR spectrum of the molecule is then calculated, and is shown below: DOI:10042/23317
product 17: 1H spectrum
Lit.shift Shift (ppm) Degeneracy Atoms 5.91 5.9217664824 1.0000 33 3.00-2.70 3.2101580044 4.0000 37,39,38,36 3.00-2.70 2.7699772356 2.0000 23,34 2.70-2.35 2.6667909953 1.0000 24 2.70-2.35 2.5087415624 1.0000 31 2.70-2.35 2.3446718332 1.0000 32 2.20-1.70 2.1808061721 2.0000 42,43 2.20-1.70 2.0641651133 3.0000 29,40,44 2.20-1.70 1.9345891291 2.0000 41,30 2.20-1.70 1.7988723289 2.0000 35,28 2.20-1.70 1.7400613561 1.0000 25 1.58 1.6891138864 1.0000 27 1.50-1.20 1.5001282125 2.0000 47,50 1.50-1.20 1.3341332783 1.0000 49 1.50-1.20 1.1952694562 1.0000 48 1.07 1.0998205045 1.0000 26 1.03 0.9971192065 2.0000 46,45
product 17: 13C spectrum]
Lit. shift Shift (ppm) Degeneracy Atoms 211.49 212.50 1.0000 8 148.72 148.03 1.0000 16 120.9 124.402 1.0000 9 74.61 90.02 1.0000 6 60.53 58.37 1.0000 2 51.3 55.60 1.0000 15 50.94 52.83 1.0000 1 45.53 49.77 1.0000 19 43.28 46.81 1.0000 5 40.82 44.41 1.0000 13 38.73; 36.78 43.85 2.0000 10,12 30.00 32.19 1.0000 3 25.56 30.81 1.0000 7 25.35 27.95 1.0000 4 22.21 27.66 1.0000 18 21.39 25.61 2.0000 21,17 1983 23.66 1.0000 22
As a general whole, there appear to be good consensus between the literature[5] and calculated chemicial shifts value; with deviations being mostly within ±5ppm. With the proton NMR, the experimental literature value is a lot more vague, this is becuase the environments are very similar and may have caused the peaks to have overlapped. Furthermore, the solvent used for the literature values are C6D6 rather than the CDCl3 used in the calculations. Therefore, ideally the calculations should be redone with the C6D6 solvent.
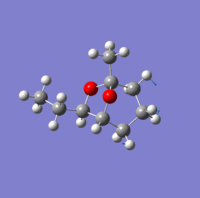
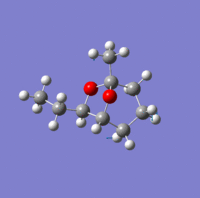
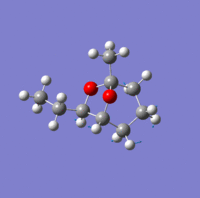
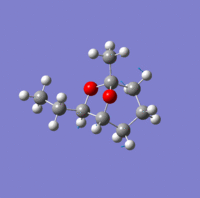
Investigation of exo- and endo- Brevicomin
the equivalence of 5 billion board feet of timber are killed every year by bark beetles, through a two phase process. An initial attack of a small number of beetles. followed by a massive second wave, which leads to the death of the tree. The initial beetles would bore into the tree and construct a chamber, in which they will release an attractant that will lead to the triggering of the secondary invasion. This attractant is produced by the female to attract males.
Exo-brevicomin is the principal sex attractant of the western pine beetle Dendroctonus brevicomis; with an unusual 6,8-dioxabicyclo[3.2.l]octane structure. The chemical could be used to manipulate the mating habits of the beetles, which would provide an ecological advantageous way of controlling the population of these destructive insects. A stereo-selective synthesis of both the exo and endo isomer has been developed through the addition of an intermediate; that has the sufficient flexibility to permit conversion between the two isomers.
Optimization
The molecule was first optimized by the MM2 forcefield:
| Endo-isomer(kcal/mol) | exo-isomer(kcal/mol) | Difference (kcal/mol) | |
|---|---|---|---|
| Stretch | 1.1843 | 1.1866 | -0.0023 |
| Bend | 4.6834 | 3.2589 | 1.4245 |
| Stretch-bend | 0.3965 | 0.3474 | 0.0491 |
| Torsion | 12.5558 | 12.1678 | 0.388 |
| Non - 1,4 VDW | -2.3494 | -2.4187 | 0.0693 |
| 1,4 VDW | 11.7242 | 11.7065 | 0.0177 |
| Dipole/dipole | 2.3790 | 2.5775 | -0.1985 |
| Total energy | 30.5738 | 28.8261 | 1.7477 |
There is not a very big difference between the two molecule; with the largest contribution to the difference being the bend energy. For the exo-isomer there is a smaller level of steric hindrance directly underneath the ring; therefore less energy is required for it to bend. in addition, the ethyl group attached to the oxygen is pointing away from the molecule, meaning that there is also less steric hindrance. As a result, the exo -isomer is slightly lower in energy.
Another calculation which can be used to investigate the energy is through Gaussian. The conditions set are: Calculation type: RmPW1PW91 Calculation methods: 6.31G(d,p)
The energy obtained for the endo-brevicomin is -502.94903391au[6] and for exo-brevicomin it is -502.95159767au[7]. Again there is only a slight difference in energy present between the two molecules. However, there is a significant difference in the dipole moment, with 1.1817 debye for the endo-brevicomin and 1.1459debye for brevicomin. (will be investigated in the NBO analysis)
NMR
Calculated under: # mpw1pw91/6-31(d,p) NMR scrf(cpcm,solvent=chloroform)
Endo isomer [8]
<sup>13</sup>C NMR (Lit:CDCl3, 75 MHz) Lit.Shift(ppm) Calc. Shift (ppm) Degeneracy Atoms 106.99 104.2775 1.0000 4 81.68 77.8021 1.0000 7 76.53 73.4974 1.0000 6 34.44 37.6749 1.0000 3 24.99 26.1387 1.0000 11 23.63 25.7045 1.0000 9 21.88 22.356 1.0000 1 17.52 16.7045 1.0000 2 10.93 14.0148 1.0000 10
<sup>1</sup>H NMR (lit:CDCl3, 300 MHz)
Lit Calc.Shift (ppm) Degeneracy Atoms
4.22 (m, 1 H) 4.1661000000 1.0000 18
4.00 (dt, J 7.0, 4.0, 2 H 3.6118000000 1.0000 19
1.52-2.00 (m, 8 H), ) 1.7880000000 1.0000 16
1.7058000000 1.0000 12
1.4901000000 4.0000 13,15,17,26
1.3565500000 2.0000 20,21
1.44 (s, 3 H) 1.2432333333 3.0000 14,25,24
0.99 (t, J 7.2, 3 H) 1.1386000000 1.0000 27
1.0381000000 1.0000 22
0.8182000000 1.0000 23
Overall, there are good concordant present with the literature[9] and calculated 13C NMR; with only a small difference (less than ±5ppm) between the two. For the proton NMR; there is a smaller number of environments present in the literature; this implies that some of the environments are so similar that have merged (could be viewed as the same). Therefore, a good concordant is still present between the literature and calculated and these peaks may be found if a more sensitive machine is used.
Exo isomer [10]
<sup>13</sup>C NMR (Lit: CDC13) Lit. Shift (ppm) Degeneracy Atoms 107.7 105.2442 1.0 4 81.2 83.2617 1.0 7 78.3 76.1077 1.0 6 35.0 37.4281 1.0 3 29.7 31.0632 1.0 10 28.6 30.429 1.0 1 25.1 27.6965 1.0 11 17.2 17.5016 1.0 2 10.9 12.7085 1.0 9
<sup>1</sup>H NMR
Lit. Shift (ppm) Degeneracy Atoms
4.13 (1 H, br s) 4.0142000000 1.0000 18
3.93 ( 1 H, t, J 5.7) 3.5490000000 1.0000 19
1.95-1.42 (8 H, m) 2.0628000000 1.0000 12
1.8209000000 1.0000 16
1.5620000000 2.0000 26,17
1.4929000000 1.0000 15
1.41 (3 H, s) 1.3154400000 5.0000 25,23,14,13,24
1.2105000000 1.0000 22
0.91 (3 H. t, J 7.5) 1.1152000000 1.0000 27
0.8711000000 1.0000 20
0.6924000000 1.0000 21
For the carbon NMR, there is a relatively good concordance between literature[11] [12]and calculated, with about approximately a ±2ppm difference between any two values. Whereas, the proton nmr shows a larger difference between experimental and the computed results, which may suggest that a more complex basis set should be used instead. Similar to the previous results, some of the peaks predicted in the calculation have merged together in the literature results, this would be due to how similar their environment is. Therefore, to investigate these peaks fully, a more in-depth NMR analysis should be completed, through using a more sensitive machine or by lowering the temperature significantly.
Comparison
| Endo | Exo | |
|---|---|---|
| carbon NMR |  |
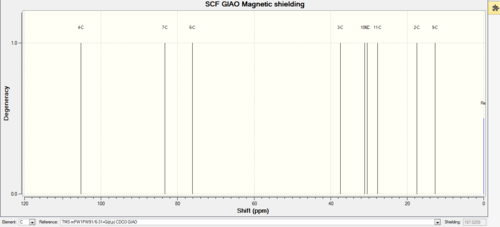
|
| Proton NMR | 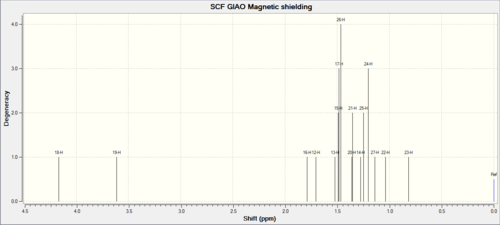 |
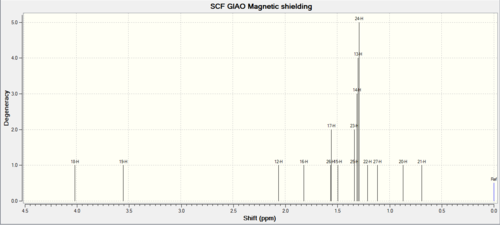
|
In general, the carbon and proton nmr for the isomers looks relatively similar to each other. For the carbon NMR; the exo isomer has slighty larger chemical shifts value than the endo-isomer. Furthermore, the chemical shift value has changed for atom 10 and 9 between the two atoms. For the endo-isomer, carbon 10 is 14.0148ppm and carbon 9 is 25.7045ppm; whereas it is carbon 10 is 31.0632ppm and carbon 9 is 12.7085ppm for the exo. This can be used as a indication of which are present within the system; providing a sensitive enough machine is used.
For the proton NMR; in general the exo-ismoer is lower in chemical shift than the endo one. Again there are small differences which could be used to establish the difference between the two.
IR
Conditions: b3lyp/6-31G(d,p) opt freq
For endo-isomer[13] for sum electronic and thermal free energies is -502.853616; whereas it is -502.856505 for the exo[14].
The literature value[15] of 2854cm-1 can not be identified in the calculated vibrations. Furthermore there is a greater difference between the literature and calculated for the larger wavelengths. This may be because larger wavelengths are generally stretched, which were calculated with a considerable greater margin of error than the same bending wavelengths.
Although 2940 and 2878cm-1 are present in the literature[16], there are no predicted vibrations between 1528 and 3026 cm-1. Which may suggest that the calculation may be incorrect or not to a sufficient level of accuracy. Therefore, a better basis set should be attempted.
As a whole, there are good concordance between literature and calculated for the smaller vibrations. However, there are larger difference between the larger vibrations. This may be due to the fact that the error for stretching modes are considerably higher than those for bends.
comparison
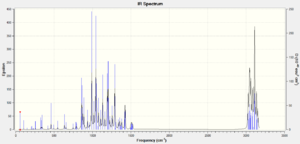
To compare the IR easily, the two IR spectrums considered. Broadly speaking, they are both very similar and contain two groups of peaks. However the endo iosmer contain a strong peak at just below 3000cm-1; which does not appear in the other. However, through IR alone, it will be near impossible to establish which spectrum belong to which isomer as they are too similar.
.
Additional analysis
Optical rotation There are three chiral centres present in this molecule, with only a difference one between the endo[17] and exo [18] isomer.
For the endo: [Alpha] ( 5890.0 A) = 17.24 deg. For the exo: [Alpha] ( 5890.0 A) = 95.01 deg. Ideally, the result expected is for the two value to be similar; with one being negative and another positive. Which would indicate that they are enantiomeric to each other. This may be indicative that the calculations were performed poorly and better basis group may be needed.
UV-Vis AND CD (Circular Dichroism) Spectrum
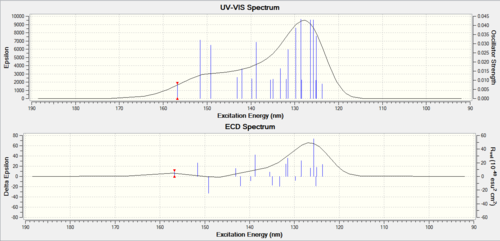
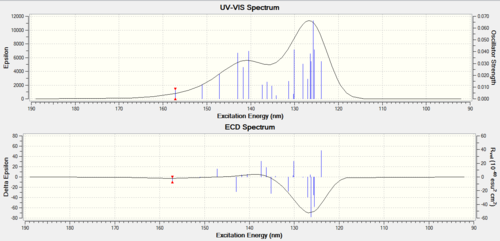
UV-Vis is the same for the endo [19] and exo[20]DOI:10042/23312 </ref>}}</ref> molecule - but their CD spectrum are different, with one as a peak and another as a trough. As there is a clear difference exhibited- this is a good method to use to distinguish between the two.
Mechanism
Both the endo and exo isomer can be prepared through the formation of the same intermediate. The acetylenic ketal;(4) is able to stereaselectively reduce the asetylenes into either its cis or trans alkene.
The exo brevicomin(8) can be prepared to an overall 42% yield through a three step process from the intermediate. Through the reduction of the acetylene with BHS.Me2S, then protonolysis to give the cis olefin. Which can then oxidised to form an epoxide; followed by a stereo-specific acid-catalysed cleavage of the epoxide with con-comitant hydrolysis of the ketal function
Endo-brevicomin can also be prepared through a three step process to produce a higher yield of 77%. First, by the NaNH3 reduction of acetylenic ketal, followed by an acid hydrolysis. This would produce the intermediate erythro keto diol (11), which would be able to cyclise under the reaction condition.
side note
The exo isomer (retention time 14.6 min) was contaminated with <1% of the endo isomer 12 (re- tention time 20.2 min) by VPC (10 ft X 0.25 in. 10% Carbowax 2000 on Chromosorb W, 150°, He flow 20 ml/min). Analysis by VPC (10 f t X 0.25 in. 10% Carbowax 2000 on Chromosorb W, 150°, He flow 20 ml/min) showed endo-brevicom- in (retention time 20.2 min) contaminated with <1% of the exo iso- mer (retention time 14.6 min).
Instead of analyzing through spectrum, another method would be to consider the retention time of the isomer[22]. As this shows clearly that one adsorp more strongly than the other; leading to a difference in retention time.
Conclusion
The compounds were successfully analysed by Chem bio3D and Gaussian. (And i have successfully - to a degree- survived through computational labs >.<!)
reference
- ↑ Skala, D; Hanika, J. Kinetics of Dicyclopentadiene hydrogenation using Pd/C Catalyst. Petroleum and coal, Vol. 45, 3-4, 105-108
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- ↑ D. M. Whitfield, T. Nukada, Carbohydr. Res., 2007, 342, 1291. DOI:10.1016/j.carres.2007.03.030
- ↑ L. Paquette, N. A. Pegg, D. Toops, G. D. Maynard, R. D. Rogers, J. Am. Chem. Soc.,, 1990, 112, 277-283. DOI:10.1021/ja00157a043
- ↑ DOI:10042/23314
- ↑ DOI:10042/23307
- ↑ DOI:10042/23315
- ↑ Gallos, J.K; Kyradjoglou, L.C.; Koftis, T.V. A concise synthesis of (-)-endo-Brevicomin. HETEROCYCLES, Vol. 55, No. 4, 2001, pp. 781 – 784.
- ↑ DOI:10042/23306
- ↑ Pal, P;Shaw, A.k. Stereoselective total syntheses of (þ)-exo- and ( )-exo-brevicomins,(þ)-endo- and ( )-endo-brevicomins, (þ)- and ( )-cardiobutanolides,(þ)-goniofufurone. Tetrahedron 67 (2011) 4036e4047]
- ↑ Zhang, J.C; Curran, D.P. Stereoselective Synthesis of I ,2-Diols by the Cycloadditive Strategy: Total Synthesis of ( +)-exo-Brevicomin and ( + ) - and (-)-Pestalotin. J. C H E M . soc-. P E R K I N TRANS. I 1991
- ↑ DOI:10042/23311
- ↑ DOI:10042/23305
- ↑ Pal, P;Shaw, A.k. Stereoselective total syntheses of (þ)-exo- and ( )-exo-brevicomins,(þ)-endo- and ( )-endo-brevicomins, (þ)- and ( )-cardiobutanolides,(þ)-goniofufurone. Tetrahedron 67 (2011) 4036e4047]
- ↑ Zhang, J.C; Curran, D.P. Stereoselective Synthesis of I ,2-Diols by the Cycloadditive Strategy: Total Synthesis of ( +)-exo-Brevicomin and ( + ) - and (-)-Pestalotin. J. C H E M . soc-. P E R K I N TRANS. I 1991
- ↑ DOI:10042/23309
- ↑ DOI:10042/23304
- ↑ DOI:10042/23310
- ↑ {{DOI|
- ↑ Kocienski P,J; Ostrow, R.W. A Stereoselective Total Synthesis of exo- and endo-Brevicomin. 8 J. Org. Chem., Vol. 41, No. 2, 1976
- ↑ Pal, P;Shaw, A.k. Stereoselective total syntheses of (þ)-exo- and ( )-exo-brevicomins,(þ)-endo- and ( )-endo-brevicomins, (þ)- and ( )-cardiobutanolides,(þ)-goniofufurone. Tetrahedron 67 (2011) 4036e4047]

![Mechanism of endo and exo isomer[21]](/images/0/07/Mechanism.PNG)