Rep:Mod:syc2001
Aromaticity
Shuk Yi Chan
Introduction
In this report the aromatic compounds benzene, boratabenzene, pyridinium and borazine and will be investigated through considering the vibrations, molecular orbitals and NBO analysis.
NOTE: the structure of borazine is incorrect in this report.
benzene
Optimization and frequency of benzene
Benzene is optimised with the bais set 6.31G(d,p)in order to find the most stable form. Link DOI:10042/22789
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6.31G(d,p) |
| Final Energy (au) | -232.25820412 |
| Gradient (au) | 0.00009093 |
| Dipole Moment (debye) | 0.0000 |
| Point Group | C1 |
| Calculation Time | 3 minute 17.9 seconds |
Item Value Threshold Converged?
Maximum Force 0.000198 0.000450 YES
RMS Force 0.000082 0.000300 YES
Maximum Displacement 0.000849 0.001800 YES
RMS Displacement 0.000305 0.001200 YES
Predicted change in Energy=-4.741797D-07
Optimization completed.
-- Stationary point found.
To ensure that the obtained molecule is the lowest energy form, the frequency is investigated. link DOI:10042/22788
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6.31G(d,p) |
| Final Energy (au) | -232.25820450 |
| Gradient (au) | 0.00000378 |
| Dipole Moment (debye) | 0.0001 |
| Point Group | C1 |
| Calculation Time | 10 minute 38.4 seconds |
Low frequencies --- -0.0007 -0.0003 0.0005 1.3671 4.9232 5.9813 Low frequencies --- 414.5185 414.6191 621.0655
The frequency is very close to zero and the frequency range is within the 15cm-1. Therefore the optimised C-C and C-H bond length are 1.39633Å and 1.08634 respectively.
Molecular Orbitals and NBO of benzene
the Molecular Orbitals and NBO are obtained for benzene Link DOI:10042/22790
| File Type | .fch |
| Calculation Type | SP |
| Calculation Method | RB3LYP |
| Basis Set | 6.31G(d,p) |
| Final Energy (au) | -232.25821841 |
| Gradient (au) | 0.0000 |
| Dipole Moment (debye) | 0.0000 |
| Point Group | C1 |
| Calculation Time | 1 minute 30.1 seconds |
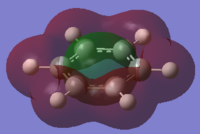
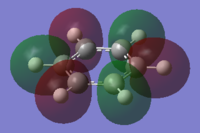
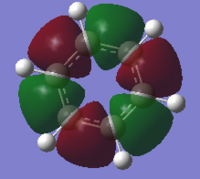
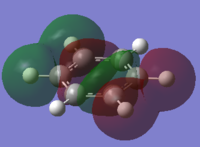
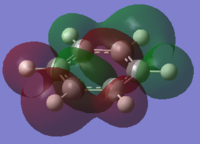
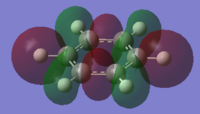
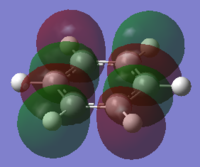
the Molecular orbitals that would be considered are between 7 and 30:
| HOMO-13 | HOMO-12 | HOMO-11 | HOMO-10 | HOMO-9 | HOMO-8 |
 |
 |
 |
 |
 |
|
| HOMO-7 | HOMO-6 | HOMO-5 | HOMO-4 | HOMO-3 | HOMO-2 |
 |
 |
 |
 |
 |
|
| HOMO-1 | HOMO | HOMO | LUMO | LUMO | LUMO+1 |
 |
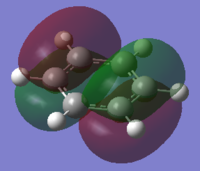 |
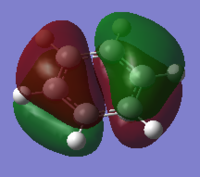 |
 |
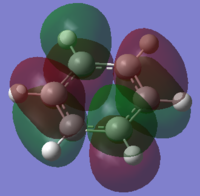 |

|
| LUMO+2 | LUMO+3 | LUMO+4 | LUMO+5 | LUMO+6 | LUMO+7 |
 |
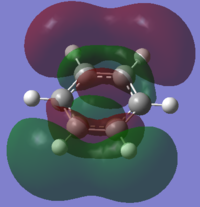 |
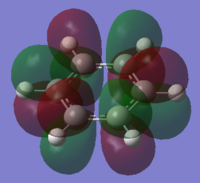 |
 |
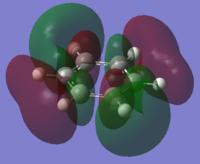 |
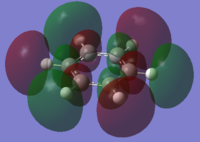
|
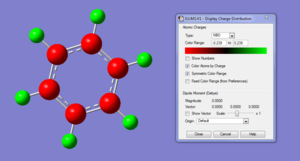

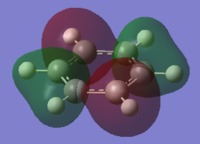
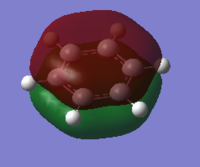
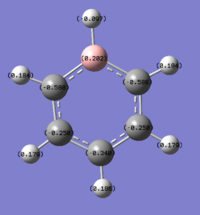
The charge distribution of the benzene ring shows a colour range between -0.239 to 0.239. Bright green indicates highly positive charge and bright red highly negative charge. There is a high negative charge on the carbon atoms, suggesting that there is a high level of electron density, which is consistent with the idea of an electron rich aromatic ring. the numbers suggest that the high electron density is shared equally between the 6 carbon, and are withdrawn equally from the hydrogen.
.
Analysis

One of concept of aromaticity is that the system tends to be more stable when the electrons are more dispersed, or more delocalised; as this leads to a high increase in stabilization energy. This can be shown by the pi orbitals in the molecular orbital diagrams, as they are shown to have electron density spread across the aromatic ring. The sigma orbitals shows how the rest of the system are bonded together, these have a high stabilisation energy suggesting that it is goo A molecule can be defined as aromatic if it is cyclic, planar with a conjugated π system of 4n+2 electrons. For benzene, there are 6 electrons in the aromatic ring, with each single electron being a single unpaired electron in the p orbital. Generally, only these electrons are considered in the aromaticity – with them being the orbitals 17, 20-23 and 27. Whereas all the sigma orbitals are all perpendical to the π electrons.
Boratabenzene
Optimization and frequency
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | UB3LYP |
| Basis Set | 6.31G(d,p) |
| Charge | -1 |
| Spin | singlet |
| Final Energy (au) | -219.02052178 |
| Gradient (au) | 0.00017317 |
| Dipole Moment (debye) | 2.8495 |
| Point Group | C1 |
| Calculation Time | 6 minute 42.1 seconds |
Item Value Threshold Converged?
Maximum Force 0.000433 0.000450 YES
RMS Force 0.000086 0.000300 YES
Maximum Displacement 0.001784 0.001800 YES
RMS Displacement 0.000524 0.001200 YES
Predicted change in Energy=-1.167402D-06
Optimization completed.
-- Stationary point found.
the gaussian frequency calculation is used to verify that the obtained structure is the minimum energy form.
Link DOI:10042/22839
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6.31G(d,p) |
| Charge | -1 |
| Spin | singlet |
| Final Energy (au) | -219.02052299 |
| Gradient (au) | 0.00000111 |
| Dipole Moment (debye) | 2.8455 |
| Point Group | C1 |
| Calculation Time | 10 minute 35.9 seconds |
Low frequencies --- -7.1651 -0.0006 0.0003 0.0003 3.3462 4.7101 Low frequencies --- 371.2978 404.4172 565.0786
MO and NBO
| File Type | .fch |
| Calculation Type | sp |
| Calculation Method | RB3LYP |
| Basis Set | 6.31G(d,p) |
| Charge | -1 |
| Spin | singlet |
| Final Energy (au) | -219.02052932 |
| Gradient (au) | 0.000 |
| Dipole Moment (debye) | 2.8494 |
| Point Group | (not listed) |
| Calculation Time | (not listed) |
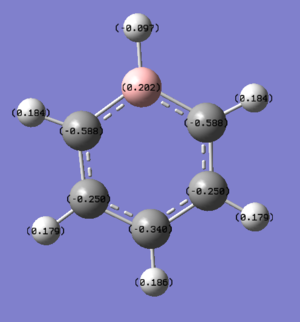
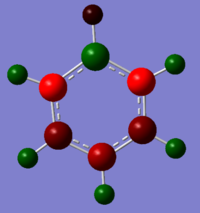
Unlike benzene, the electron density does not spread equally across the aromatic ring, as the symmetry in the system has been disrupted.The Boron atom has a charge of 0.202 and is coloured green, which suggest that there it is electropostive and there is a low level of electron density. The hydrogen atom attached to the B atom is considerable more electron rich than the other hydrogen, this is because it is able to withdraw electron from the Boron (hydrogen has a higher electronegativity value compared to boron). Finally, the two carbon atoms adjacent to the boron has the lowest charge value, because there is a highest level of electron density; as they are able to withdraw it from both the attached hydrogen atom and the boron.
.
Pyridinium
Optimization and Frequecies

| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6.31G(d,p) |
| Charge | 1 |
| Spin | singlet |
| Final Energy (au) | -248.66806084 |
| Gradient (au) | 0.00003928 |
| Dipole Moment (debye) | 1.8728 |
| Point Group | C1 |
| Calculation Time | 4 minute 30.2 seconds |
Item Value Threshold Converged?
Maximum Force 0.000065 0.000450 YES
RMS Force 0.000023 0.000300 YES
Maximum Displacement 0.000832 0.001800 YES
RMS Displacement 0.000177 0.001200 YES
Predicted change in Energy=-7.041043D-08
Optimization completed.
-- Stationary point found.
the frequency of the molecules is then calculated. link DOI:10042/22836
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6.31G(d,p) |
| Charge | 1 |
| Spin | singlet |
| Final Energy (au) | -248.66806092 |
| Gradient (au) | 0.00000190 |
| Dipole Moment (debye) | 1.8723 |
| Point Group | C1 |
| Calculation Time | 8 minute 52.3 seconds |
Low frequencies --- -9.4157 -3.1359 -0.0010 -0.0006 -0.0006 0.8682 Low frequencies --- 391.8996 404.3408 620.2003
the frequency are within a range of 15-1 and there optimization has been successful. With the length for the C-H, C-C, C-N and N-H bonds are 1.08524, 1.39873, 1.35242angstroms.
MO and NBO
| File Type | .log |
| Calculation Type | SP |
| Calculation Method | RB3LYP |
| Basis Set | 6.31G(d,p) |
| Charge | 1 |
| Spin | singlet |
| Final Energy (au) | -248.66806084 |
| Gradient (au) | 0.0000 |
| Dipole Moment (debye) | 1.8729 |
| Point Group | C1 |
| Calculation Time | 1 minute 36.2 seconds |
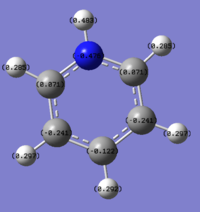
The colour range from green(positive electronic charge) to red (negative electron charge0; with a range of -0.483 to 0.483.
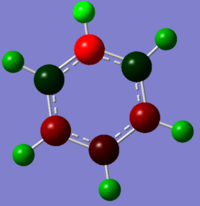
The nitrogen atom has a very high negative charge, which corresponds well to the knowledge that it is highly electronegative and will withdraw electron density from its adjacent atoms; as its electronegativity value being much higher than both the carbon and hydrogen atom.Resulting the two adjacent carbon atoms having slightly positive charge (0.071) and the hydrogen atom having a charge of 0.483 (compared to the hydrogen attached to the carbon having a charge of 0.285).
.
Borazine
Optimization and Frequecies
Link {DOI|10042/22867}}
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6.31G(d,p) |
| Final Energy (au) | -272.41804280 |
| Gradient (au) | 0.00001299 |
| Dipole Moment (debye) | 0.1711 |
| Point Group | C1 |
| Calculation Time | 18 minute 3.1 seconds |
Item Value Threshold Converged?
Maximum Force 0.000033 0.000450 YES
RMS Force 0.000010 0.000300 YES
Maximum Displacement 0.001006 0.001800 YES
RMS Displacement 0.000178 0.001200 YES
Predicted change in Energy=-1.958746D-08
Optimization completed.
-- Stationary point found.
frequency calculations.
Link {DOI|10042/22867}}
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6.31G(d,p) |
| Final Energy (au) | -272.41804283 |
| Gradient (au) | 0.00000092 |
| Dipole Moment (debye) | 0.1716 |
| Point Group | C1 |
| Calculation Time | 11 minute 2.1 seconds |
Low frequencies --- -4.5494 -3.2512 0.0005 0.0007 0.0009 3.3543 Low frequencies --- 237.8920 321.4218 363.2847
the frequency are relatively to 0 and are within the 15cm-1 range.
MO and NBO

| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6.31G(d,p) |
| Final Energy (au) | -272.41804280 |
| Gradient (au) | 0.00001299 |
| Dipole Moment (debye) | 0.1711 |
| Point Group | C1 |
| Calculation Time | 18 minute 3.1 seconds |
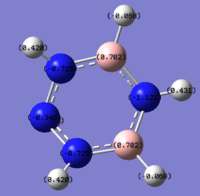
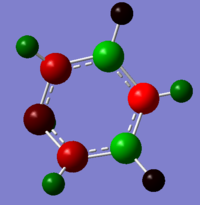
The nitrogen atom adjacent to both of the boron atom has the highest negative charge and largest amount of electron density; this is because the electron-negative nitrogen is able to withdraw electron density from two boron atoms and the hydrogen atom. The two nitrogen atoms with a charge of -0.725 have a lower charge is because each of the nitrogen atom can withdraw electron density from only one adjacent boron atom. With the final nitrogen of -0.349 charge being only able to withdraw electron from the hydrogen bond. All four nitrogen appear to withdraw an equal proportion of electron density from their adjacent hydrogen bond, as the charges between them are relatively similar. Finally the hydrogen on the boron are slightly electron negative because it has a higher electronegativity value compared to boron.
.
Analysis
Charge distribution Comparisons
| Benzene | Boratabenzene | pyridinium | Borazine |
|---|---|---|---|
 |
 |
 |
|
 |
 |
 |

|
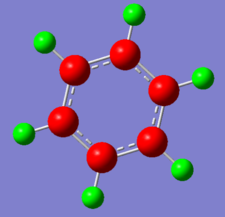
| -0.239 to 0.239 | -0.588 to 0.588 | -0.483 to 0.483 | -1.121 to 1.121 |
The calculated charge distribution is shown above, with all these diagram, the colour red indicate a negative charge density; and hence the positive charge density is shown by the green colour. With the bottom row being the charge range.
Only benzene has a symmetrical charge density diagram, as the structure has D6h structure with the electron density being spread equally across the aromatic ring. However, by changing a carbon atom with something that is either higher or lower in electronegativity (addition of polarity leads to dipoles in the system); there will be regions of high and low electron density across the aromatic ring. For boratabenzene, there are some similarities with the Benzene charge distribution analysis; the carbon atoms are electron rich whilst the hydrogen atoms are relatively electron poor. However, boron is able to donate electrons to its adjacent atoms, resulting in the two adjacent carbon atoms gaining a high electron density. The total charge density in the system equaling to -1.
Whereas for the pyridinium ion, there is a reverse effect compared to the boratabenzene. This is because boron has a lower electronegativity value compared to the other atoms in the system; whereas the nitrogen atom is significantly more electronegative than both the carbon and hydrogen. Therefore, rather than donating electrons; the nitrogen atom is withdrawing electron density away from the adjacent atoms. Leading to the adjacent carbon and hydrogen atoms in having a higher positive charge density compared to those further away from the nitrogen atom. It can be argued that pyridinium and benzene are the two extreme, one system containing an electro-withdrawing atom and the other containing an electron donating; with benzene and borazine lying in between. Benzene’s electron density are spread equally across the 6 atoms; whereas with borazine there are regions of high and low electron density with respect to the atom. The nitrogen atom has a low negative charge as it is able to pull electron density away from its adjacent less electronegative atoms, leading to positive charges on the nitrogen and adjacent hydrogen.
Molecular Orbitals
| Benzene | Boratabenzene | pyridinium | Borazine |
|---|---|---|---|
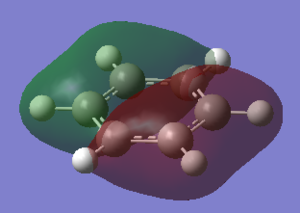 |
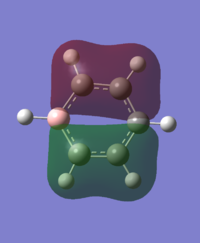 |
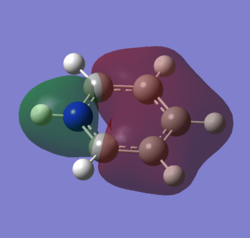 |
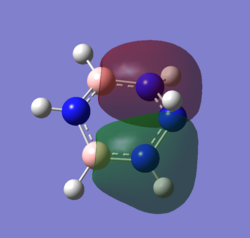
|
 |
 |
 |
|
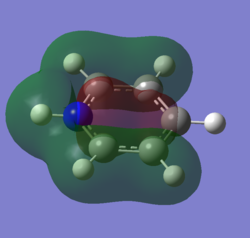
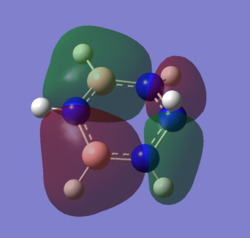
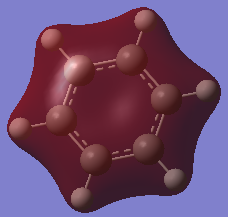
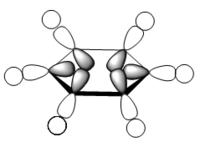
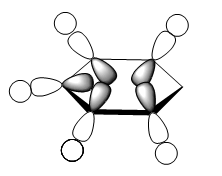
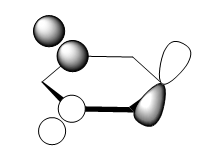
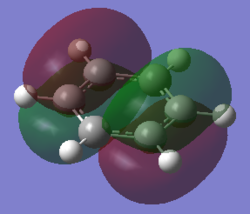
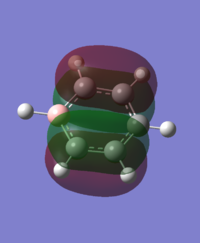
For the molecular orbital 8, all four molecules share some similarities, with the molecular into two phases. For boratabenzene, benzene and borazine, these phases are approximately around the same size with respect to each other. However, for pyridinium, the phase around the nitrogen atom is significantly smaller than the other which spread across the rest of the aromatic.
| Benzene | Boratabenzene | pyridinium | Borazine |
|---|---|---|---|
 |
 |
 |
|
 |
 |
 |
|
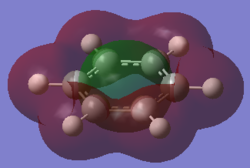
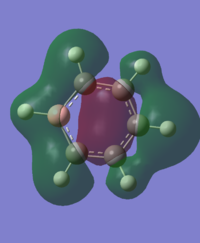
For the molecular orbital 12, there are similarities between the first three diagrams. However, for the boratabenzene and pyridinium, the phase has reversed in comparison to benzene, and the ring is broken. With the ring being broken in two places for the boratabenzene and only one for the pydridium. Contrastingly, the molecular orbital for borazine is very different, with no ring present in the diagram.
| Benzene | Boratabenzene | pyridinium | Borazine |
|---|---|---|---|
 |
 |
 |
|
 |
 |
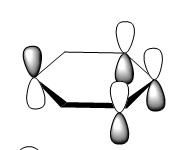 |
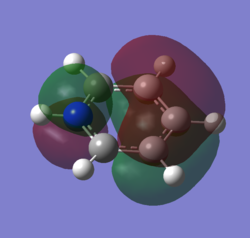
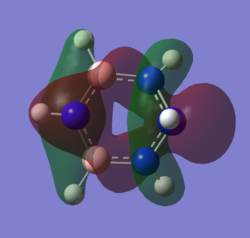
The molecular orbital 20 is a bonding π orbital, which is also one of the homo of the system. The electrons are perpendicular to the planar benzene ring, therefore this orbital contribute to the conjugated aromatic π system. For benzene and boratabenzene, the molecular orbital are relatively similar, as the generally the orbitals have good orbital overlap. Unlike boratabenzene, the pyridinium’s heter-atom is contributing to the molecular orbital; leading to a decrease of orbital size on the nitrogen side of the molecule and an increase for the opposite. This is due to the nitrogen atom ability to withdraw electron density, leading to a smaller orbital being formed. Whereas, the borazine’s molecular orbital is very different compared to the other three.
LCAOs Contributing to Molecular orbitals.
For benzene, the atomic orbital contribution in the molecular orbital would be equal, as there are only carbon and hydrogen atoms; which would lead to a overlap of orbitals of equal sizes and energy. However, with heteraromatic, these atomic orbitals are no longer equivalent, as they boron and nitrogen do not have the same energy to carbon or each other. In general, atomic orbitals of similar energy gives the highest level of stabilization energy, therefore the combination of carbon and heter-atoms leads to poorer overlap and less stabilization energy.
Whereas, for borazine, there are two groups of atomic orbitals which have the same energy (2 degenerate set of AO from B and 4 degenerate set from the nitrogen atom). The overlap of these orbitals would be relatively poor, as there is a greater difference in electronegativity (compared to C with B and C with N.)
Energy of Mos
Atomic orbitals with a small energy difference and relatively similar sizes would lead to a large stabilization energy in the molecular orbital. For benzene, the stabilization energy is very good, as the same size and energy orbitals lead to a high level of overlap . Whereas, the electronegativity leads to a energy difference between the atomic molecules. However, the orbital size between N, C and B are similar as they are all in the 2S and 2P shell. The largest energy difference would be largest for borazine because there is a greatest difference in electronegativity, and therefore lead to the smallest stabilization energy.
ordering of Molecular orbitals
For borazine there is ordering present in the system, which does not occur for the other three systems. This is due to the electronegativity of the system, which leads to poorer overlap between some of the atomic orbitals. As some of the molecular orbitals remained constant; the atomic orbitals has a such a low stabilization energy that they have surpassed these orbitals in energy. Leading to a reordering of the system. This really only occurs with borazine, as there is not a sufficient electronegative difference between the atomic orbitals in pyridinium and boratabenzene.
Degeneracy of Molecular orbitals
Benzene is a very symmetrical molecule, therefore there is a high level of degenerate molecular orbitals. However the addition of B leads to a disruption in the symmetry would lead to a decrease in degeneracy. (There are only a few degenerate orbitals present in the system). Whereas, the borazine and pyridinium, there are no degeneracy at all present.
Degeneracy of Molecular orbitals
Benzene is a very symmetrical molecule, therefore there is a high level of degenerate molecular orbitals. However the addition of B leads to a disruption in the symmetry would lead to a decreasing level of degeneracy. (There are only a few degenerate orbitals present in the system). Whereas, the borazine and pyridinium, there are no degeneracy at all present.
effect of substitution on the full molecular orbital diagram
A combination of all the previous effect means that substitution leads to a decreasing in the stabilization energy of the system, loss of degeneracy and possibly a reordering of the orbitals. In these examples, the key contribution to this is the difference in electronegativity; whereas in other example atomic size and would also be a key issue.
Correction
Throughout the report, the wrong molecule was used for borazine; therefore the correct calculations would be completed here: File:BORAZINE OPTIMIZATION.LOG DOI:10042/22895
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6.31G(d,p) |
| Final Energy (au) | -242.68459989 |
| Gradient (au) | 0.00006367 |
| Dipole Moment (debye) | 0.0000 |
| Point Group | C1 |
| Calculation Time | 9 minute 0.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000084 0.000450 YES
RMS Force 0.000033 0.000300 YES
Maximum Displacement 0.000277 0.001800 YES
RMS Displacement 0.000077 0.001200 YES
Predicted change in Energy=-9.229718D-08
Optimization completed.
-- Stationary point found.
The frequency calculation is: File:Frequency.log DOI:10042/22896
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6.31G(d,p) |
| Final Energy (au) | -242.68459994 |
| Gradient (au) | 0.00000248 |
| Dipole Moment (debye) | 0.0000 |
| Point Group | C1 |
| Calculation Time | 8 minute 49.7 seconds |
Low frequencies --- -4.4401 -0.8957 -0.0015 -0.0011 -0.0006 6.8451 Low frequencies --- 289.6711 289.8164 404.5330
The low frequencies are centred around zero and is within the range of 15cm-1, confirming that the optimization had been successful.
The molecular orbital and NBO analysis: File:Bora mo.log DOI:10042/22897

| File Type | .log |
| Calculation Type | SP |
| Calculation Method | RB3LYP |
| Basis Set | 6.31G(d,p) |
| Final Energy (au) | -242.68459989 |
| Dipole Moment (debye) | 0.0000 |
| Point Group | C1 |
| Calculation Time | 1 minute 31.2 seconds |
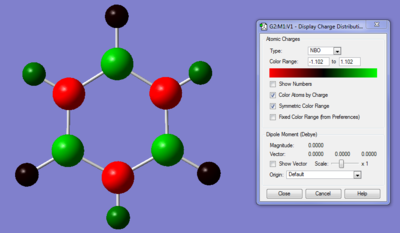
the colour range from green (high positive charge) to red (highly negative charge; with the range being from 1.102 to 1.102. the nitrogen bonds are highly electronegative, therefore they would withdraw electron density from the adjacent boron and hydrogen atoms. resulting in a highly negative charge on the nitrogen atoms and positive for the boron and adjacent hydrogen atoms. Similar to benzene,the majority of the electron density can be found on the C-C framework; however in borazine it is not split equally between the atoms.
.
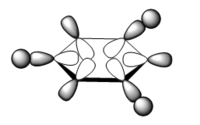
the three molecular orbitals are:
| orbital no | Computed Mo | LCAO |
|---|---|---|
| 8 |  |
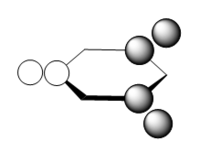
|
| 10 | 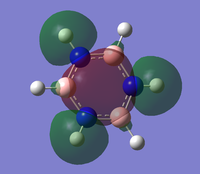 |
|
| 20 |  |
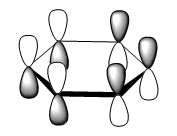
|

Note: These diagrams corresponds quite well with the other aromatics. For the molecular orbital 8. the shape is similar, however the red orbital is shaped slightly differently and is smaller; it look quite similar to the pyridinium ion. For the molecular orbital 10, it corresponds to the other three molecular orbital 12; which suggest that reordering has occurred. There is a very high level of degeneracy in the system due to the high level of symmetry in the system.
Conclusion
the four aromatic has been investigated successfully. >_<