Rep:Mod:sw4913
Gaussian Simulations of Transition States and Reactivity
Introduction
The study of transition state allows the identification of the reaction pathway undertook by the chemical reaction and the activation energy required to undergo a particular pathway. The transition state is found on a potential energy surface (PES), which is a mathematical function derived from the potential energy of molecules in terms of its position on the gradient. The first derivative of the energy of the molecule with respect to the displacement on the gradient allows the identification of a stationary point when ∂E|∂q = 0. ∂E is the change in energy of the molecule and ∂q is the displacement of the molecule, where a non-linear molecule with N atoms will have a total number of independent coordinates of 3N-6. The stationary point could be a maxima (highest energy on the PES) or a minima (lowest energy on the PES) and a second derivative of ∂E|∂q would be required to determine whether it is a maxima or a minima. A maxima will need to be observed for the transition state as it produces negative imaginary frequencies, where ∂E|∂q>0.
Three Diels Alder reactions are studied in exercises 1, 2, and 3 in a [4+2] cycloaddition manner. The transition states of these reactions were characterised using Gaussian and optimised using PM6 configurations. For exercise 2, B3LYP/6-31G(d) was also used after PM6 method to increase the accuracy of the result.
Nf710 (talk) 12:50, 24 February 2017 (UTC) This definition is too brief. A TS is a maximum in all directions apart from the reaction co-ordinate. This should be explained in second derivatives.
Exercise 1
A [4+2] cycloaddition of butadiene and ethene was studied at the PM6 level. This involves the addition of a conjugated 4π diene to a 2π dienophile to form a cyclohexene, making 2 new σ-bonds and breaking 2 π-bonds.
 |
|---|
| Figure 1: Reaction Scheme |
Molecular Orbitals
As it could be seen in figure 2 below, the HOMO of the butadiene interacts with the LUMO of ethene and the LUMO of the butadiene interacts with the HOMO of the ethene. The butadiene has a greater conjugate system hence is more stable than the ethene. The HOMO and LUMO of the interacting molecules are close in energy and hence results in great stability driving the reaction forward.
Below illustrates the interaction of the molecular orbitals of butadiene and ethene.
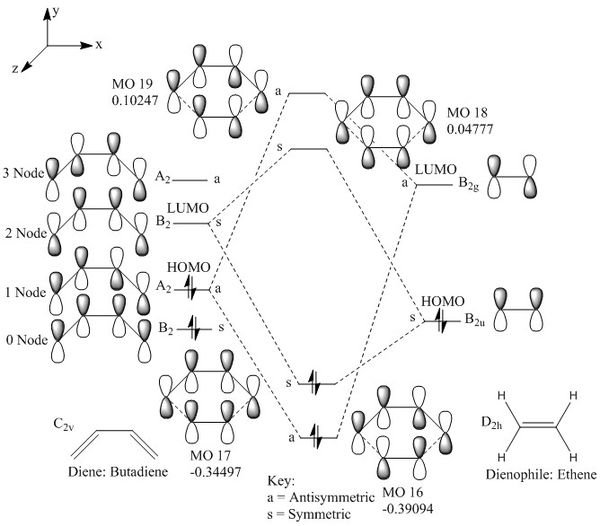 |
|---|
| Figure 2: Molecular Orbital Diagram of Reactants and Transition State |
(Fv611 (talk) 12:02, 24 February 2017 (UTC) Very nice MO diagram. Well done!)
Below are figures of the HOMO and LUMO molecular orbitals of the transition state generated by Gaussian.
|
| ||||||
|---|---|---|---|---|---|---|---|
| Figure 3: Transition State HOMO-1 (MO 16) (a,a bonding interaction) | Figure 4: Transition State HOMO (MO 17) (s,s bonding interaction) |
|
| ||||||
|---|---|---|---|---|---|---|---|
| Figure 5: Transition State LUMO (MO 18) (s,s antibonding interaction) | Figure 6: Transition State LUMO+1 (MO 19) (a,a antibonding interaction) |
Below are figures of the HOMO and LUMO MOs of the reactants.
| Figure 7: Alkene HOMO (MO 6) | Figure 8: Alkene LUMO (MO 7) |
| Figure 9: Butadiene HOMO (MO 11) | Figure 10: Butadiene LUMO (MO 12) |
The MOs calculated by Gaussian above corresponds to the theoretical MOs shown in figure 2.
It can be seen that the HOMOs and LUMOs interact together and the orbitals of the same symmetry interacts with one another. This is due to the following equation:
The integral of the orbital wavefunctions of atom A and B over space must be non-zero, which means that only the orbitals of the same symmetry can overlap, such as a symmetric-symmetric interaction and an asymmetric-asymmetric interaction is non-zero, and a symmetric-asymmetric interaction is zero.
Bond Lengths
The figure below shows the concerted cycloaddition of butadiene and ethene.
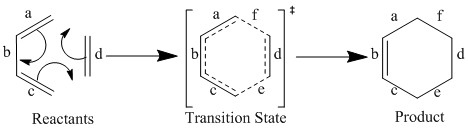 |
|---|
| Figure 11: Reaction Mechanism of the Cycloaddition of Ethene and Butadiene |
The bond lengths of a, b, c, d, e, and f were able to be explored using Gaussian calculations.
| Bond | Bond Length (Å) | ||
|---|---|---|---|
| Reactants | Transition State | Product | |
| a | 1.35531 | 1.37975 | 1.49261 |
| b | 1.46835 | 1.41093 | 1.33306 |
| c | 1.35531 | 1.37977 | 1.49261 |
| d | 1.32732 | 1.38174 | 1.53764 |
| e | na | 2.11462 | 1.53579 |
| f | na | 2.11471 | 1.53580 |
| Figure 12: Table of bond lengths | |||
The experimental values of bond length values of the product are: C(sp3)-C(sp3) = 1.54 Å, C(sp2)-C(sp2) = 1.34 Å and C(sp3)-C(sp2) = 1.50 Å. [1] These values are in close agreement with the computer generated results.
During the experiment, bond a, c, and d reduces from a double bond to a single bond, hence the length of the bonds increase, bond b increases from a single bond to a double bond decreasing the bond length. Newly bonds e and f are formed increasing the attraction between the two nuclei. The Van der Waal radii of carbon is 1.70 Å [2], which is inbetween the bond length at the transition state 2.11 Å, and the bond length of C(sp3)-C(sp3) 1.54 Å. This further confirms the concerted addition of ethene to butadiene.
(Fv611 (talk) 12:02, 24 February 2017 (UTC) In reality, the bond length at the TS (2.11) is in between the length of two Van der Waals radii (2 x 1.7) and the bond length of sp3 carbon (1.54).)
Vibrations
The vibrations below shows the vibration combining the reactants at the transition state at -948.9663 cm-1. The vibration is negative because the transition state has a negative imaginary frequency. The formation of the two bonds is synchronous.
| Figure 13: Transition state vibration |
Exercise 2
In this exercise, the cyclohexadiene and 1,3-dioxole undergoes a [4+2] cycloaddition like the above. The oxygen containing 1,3-dioxole increases the electron donating effect on the carbon making it a weaker dienophile; the cyclohexadiene acts as the diene. The reactants, products, and transition states were optimised at a PM6 first and reoptimised at the B3LYP/6-31G(d) level, an IRC calculation was carried out at the transition state. This Diels Alder reaction gives an ENDO and an EXO product as shown below in figure 14.
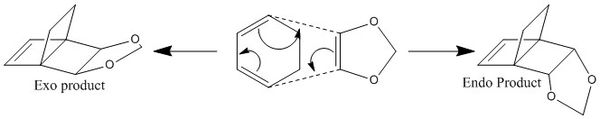 |
|---|
| Figure 14: Reaction Scheme |
Molecular Orbitals
Similarly to the MO interactions in exercise one, the HOMO and LUMO of the cyclohexadiene and 1,3-dioxole interact together. The electron donating effects of the oxygen in 1,3-dioxole destabilises the double bond and raises the energy of 1,3-dioxole, hence decreasing the energy gap of the cyclohexadiene LUMO and 1,3-dioxole HOMO and increasing the energy gap between cyclohexadiene HOMO and 1,3-dioxole LUMO. The lower energy gap of the cyclohexadiene LUMO and 1,3-dioxole HOMO meant that this is an inverse electron demand Diels Alder reaction. The interaction of the molecular orbitals of cyclohexadiene and 1,3-dioxole are shown below.
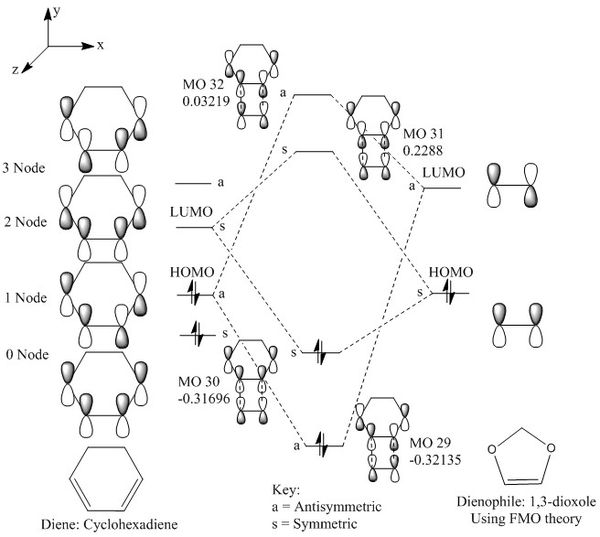 |
|---|
| Figure 15: Molecular Orbital Diagram of Reactants and Transition State |
(Wrong CHD orbital in the TS HOMO. Be careful when switching AOs around Tam10 (talk) 10:53, 21 February 2017 (UTC))
The reason that endo has a lower transition state energy barrier is because of secondary orbital interactions, where the out of plane p-orbital on the oxygen interacts with the p orbitals on the cyclohexadiene. Although this interaction is not strong enough to dominate over the interaction between the carbon atoms to form a new bond, it stabilises the endo transition structure, hence lowering the activation barrier. This interaction is not observed in the exo transition state due to the different orientation of the molecules.
The interactions of the molecular orbitals can be seen below.
Below are HOMO and LUMO orbitals for the exo transition state.
|
| ||||||
|---|---|---|---|---|---|---|---|
| Figure 16: Exo Transition State HOMO-1 (MO 40) | Figure 17: Exo Transition State HOMO (MO 41) |
| Figure 18: Exo Transition State LUMO (MO 42) | Figure 19: Exo Transition State LUMO+1 (MO 43) |
Below are HOMO and LUMO orbitals for the endo transition state.
| Figure 20: Endo Transition State HOMO-1 (MO 40) | Figure 21: Endo Transition State HOMO (MO 41) |
| Figure 22: Endo Transition State LUMO (MO 42) | Figure 23: Endo Transition State LUMO+1 (MO 43) |
It can been seen that although exo transition state has a smaller steric clash, hence lower in energy, the involvement of secondary orbital interactions in the endo transition state lowers the energy of the transition state even further.
(How did you determine sterics here? Tam10 (talk) 10:53, 21 February 2017 (UTC))
Energies
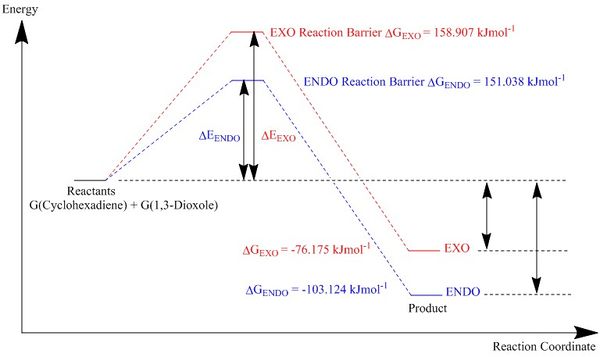 |
|---|
| Figure 24: Energy Profile of the Diels-Alder Reaction of 1,3-Dioxole and Cyclohexadiene |
A summary of the energy levels are shown below in figure 25.
| Energies (KJ/mol) | |||
|---|---|---|---|
| Exo | Endo | ||
| Reactants | -1313742.042 | -1313742.042 | |
| Product | -1313818.217 | -1313845.166 | |
| Transition State | -1313583.135 | -1313591.004 | |
| Activation Energy | 158.907 | 151.038 | |
| Change in Energy | -76.175 | -103.124 | |
| Figure 25: Table of energy levels of reactants, products, and transition states. | |||
The ENDO product is the kinetic and the thermodynamic product due to the smaller activation energy required for the transition state and the product lowest in energy.
Nf710 (talk) 14:00, 24 February 2017 (UTC) Your energies seem to be slightly. Your Activations energies are pretty much correct but your reaction energies are way off. you must have calculated them incorrectlt
Exercise 3
In this exercise, the o-xylyene and the SO2 proceeds either via the hetero Diels Alder [4+2] cycloaddition, or via the cheletropic [4+1] cycloaddition reaction. The o-xylyene acts as the diene and the SO2 acts as a dienophile. Both reaction pathways involve the breaking of 2 bonds and forming of 2 new bonds, however the Diels Alder cycloaddition proceeds with the formation of 2 new bonds on different atoms, whereas the cheletropic cycloaddition involves the formation of 2 new bonds on the same S atom. All reactions were optimised at the PM6 level. The reaction scheme summarising the Diels Alder and cheletropic reactions are shown below in figure 26.
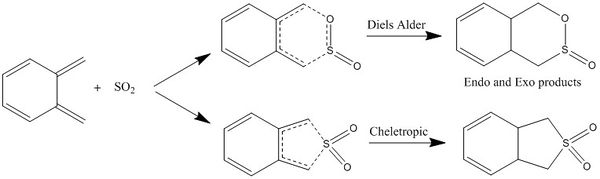 |
|---|
| Figure 26: Reaction Scheme |
Intrinsic Reaction Coordinates
The IRC of the formation of the endo transition state is shown below.

|
|---|
| Figure 27: IRC of Diels Alder ENDO Reaction |
 |
|---|
| Figure 28: IRC Calculation Pathway of Diels Alder ENDO Reaction |
The IRC of the formation of the exo transition state is shown below.

|
|---|
| Figure 29: IRC of Diels Alder EXO Reaction |
 |
|---|
| Figure 30: IRC Calculation Pathway of Diels Alder EXO Reaction |
Formation of Cheletropic product
|
|---|
| Figure 31: IRC of Cheletropic Reaction |
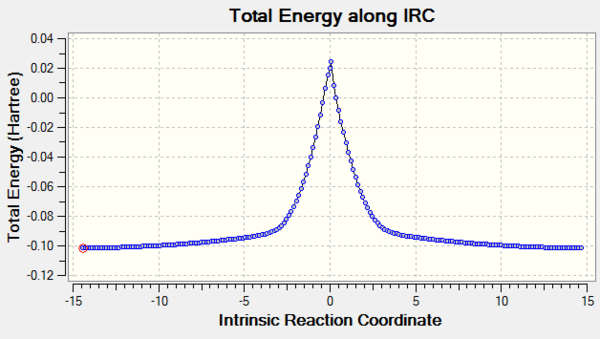 |
|---|
| Figure 32: IRC Calculation Pathway of Cheletropic Reaction |
(This IRC didn't work. You have started from a non-zero gradient - ie not the TS. This can happen when you select the wrong geometry, start from a frozen geometry or switch between QM methods Tam10 (talk) 10:53, 21 February 2017 (UTC))
It can be seen from the IRCs, that the aromatisation of the 6-membered ring across all three reactions increases the stability of the product compensating for the instability of xylylene. This stabilisation factor drives the reaction towards the products.
Energies
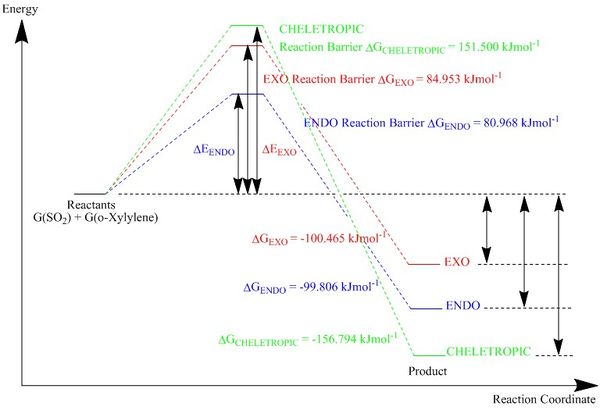 |
|---|
| Figure 33: Energy profile of the Diels Alder and Cheletropic reactions. |
(Your cheletropic energy is too high. You must always check for 1 negative frequency and a gradient of 0 in your TSs Tam10 (talk) 10:53, 21 February 2017 (UTC))
A summary of the energy levels are shown below in figure 34.
| Energies (KJ/mol) | |||
|---|---|---|---|
| Exo | Endo | Cheletropic | |
| Reactants | 156.789 | 156.789 | 156.789 |
| Product | 56.324 | 56.98 | -0.00525 |
| Transition State | 241.742 | 237.757 | 308.289 |
| Activation Energy | 84.953 | 80.968 | 151.500 |
| Change in Energy | -100.465 | -99.806 | -156.794 |
| Figure 34: Table of energy levels of reactants, products, and transition states. | |||
Similar to exercise 2, the endo product of the Diels Alder reaction is both the kinetic and thermodynamic product as it has the smallest activation energy and produces the product with the lowest energy level. This is due to the higher steric clash of the exo transition state as the two molecules approach each other.
The cheletropic reaction pathway requires the highest activation energy, but produces the most thermodynamically product out of the 3.
Conclusion
HOMO and LUMO molecular interactions of the same symmetry were studied at the transition state which has implications upon its energy levels determining whether the endo or exo product is the thermodynamic or kinetic product. In this study the endo product was found to be both the thermodynamic and kinetic product due to various reasons to do with sterics and secondary orbital interactions.
The bond lengths were also studied and showed that Gaussian calculations were a reliable representative of experimental values.
Competing reaction pathways occurred in exercise 3, although under thermodynamic control the cheletropic product would be the dominating product and under kinetic control the endo product would be the dominating product.
Other methods that can be used to study the transition state includes the use of femtosecond spectroscopy coupled with computational techniques.
References
- ↑ Bond Lengths and Energies,University of Waterloo, http://www.science.uwaterloo.ca/~cchieh/cact/c120/bondel.html, Accessed on 10.02.2017.
- ↑ S. S. Batsanov, Inorg. Mater., 2001, 37, 871–885.
