Rep:Mod:stanleyphys
Introduction
In this computational lab we will be looking into the energies, vibrations and geometries of the transition states of two different chemical reactions (The Cope rearrangement and the Diels Alder reaction) as calculated by Gaussian. By using different methods of optimisation, a transition state can be located and thus it's geometry and energies probed. Following a frequency analysis it is also possible to identify the imaginary frequency by which this reaction proceeds and also compute the molecular orbitals to examine any underlying orbital interactions that may be occurring, and thus may affect the expected reactivity.
The Cope Rearrangement
Optimising the Reactants and Products
Optimisation of anti-1,5-hexadiene
A HF/3-21G optimisation was carried out on 1,5-hexadiene with an anti-periplanar conformation for the central four C atoms using 500MB %Mem.
Link to anti 1,5-hexadiene HF/3-21G optimisation .LOG file: click here
test molecule |
Results Summary Table:
Table 1
| MS_15_hexadiene_anti_react | ||
| File Name | MS_15_HEXADIENE_ANTI_REACT | |
| File Type | .log | |
| Calculation Type | FOPT | |
| Calculation Method | RHF | |
| Basis Set | 3-21G | |
| Charge | 0 | |
| Spin | Singlet | |
| E(RHF) | -231.6926024 | a.u. |
| RMS Gradient Norm | 0.00001047 | a.u. |
| Imaginary Freq | ||
| Dipole Moment | 0.2024 | Debye |
| Point Group | C1 | |
| Job cpu time: 8 minutes 31.0 seconds | ||
Calculation Log: 'Item' table of converged forces and distances for 3-21G anti-1,5-hexadiene optimisation:
Item Value Threshold Converged?
Maximum Force 0.000024 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.001726 0.001800 YES
RMS Displacement 0.000525 0.001200 YES
Predicted change in Energy=-2.769091D-08
Optimization completed.
-- Stationary point found.
Result of Symmeterise Function: C2
Total Energy Minimum Value for 3-21G optimisation of anti-1,5-hexadiene Molecule: -231.6926024 Hartee
Optimisation of gauche-1,5-hexadiene
A HF/3-21G optimisation was carried out on 1,5-hexadiene with gauche linkage between the central four C atoms using 500MB %Mem.
Link to anti 1,5-hexadiene HF/3-21G optimisation .LOG file: click here
test molecule |
Results summary table:
Table 2
| MS_15_hexadiene_gauche_react | ||
| File Name | MS_15_HEXADIENE_GAUCHE_REACT | |
| File Type | .log | |
| Calculation Type | FOPT | |
| Calculation Method | RHF | |
| Basis Set | 3-21G | |
| Charge | 0 | |
| Spin | Singlet | |
| E(RHF) | -231.691667 | a.u. |
| RMS Gradient Norm | 0.00000956 | a.u. |
| Imaginary Freq | ||
| Dipole Moment | 0.3802 | Debye |
| Point Group | C1 | |
| Job cpu time: 4 minutes 52.0 seconds | ||
Calculation Log: 'Item' table of converged forces and distances for 3-21G gauche-1,5-hexadiene optimisation:
Item Value Threshold Converged?
Maximum Force 0.000019 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000648 0.001800 YES
RMS Displacement 0.000234 0.001200 YES
Predicted change in Energy=-1.074939D-08
Optimization completed.
-- Stationary point found.
Result of Symmeterise Function: C2
Total Energy Minimum Value for 3-21G optimisation of gauche-1,5-hexadiene Molecule: -231.691667 Hartee
Lowest Energy Conformation of 1,5-hexadiene
Since the lower in energy of the two optimisations was that of the gauche conformation of 1,5-hexadiene, it can be inferred that the lowest energy conformer is likely to have a gauche linkage between the central four carbon atoms. From this starting point optimisations were run of 1,5-hexadiene, and the following conformation was found to be the lowest in energy:
A HF/3-21G optimisation was carried out on the 'gauche 3' 1,5-hexadiene using 500MB %Mem.
Link to gauche 3 1,5-hexadiene HF/3-21G optimisation .LOG file: click here
test molecule |
Results summary table:
Table 3
| MS_15_hexadiene_gauche3_react | ||
| File Name | MS_15_HEXADIENE_GAUCHE3_REACT | |
| File Type | .log | |
| Calculation Type | FOPT | |
| Calculation Method | RHF | |
| Basis Set | 3-21G | |
| Charge | 0 | |
| Spin | Singlet | |
| E(RHF) | -231.6926612 | a.u. |
| RMS Gradient Norm | 0.00002306 | a.u. |
| Imaginary Freq | ||
| Dipole Moment | 0.3404 | Debye |
| Point Group | C1 | |
| Job cpu time: 1 minutes 37.0 seconds | ||
Calculation Log: 'Item' table of converged forces and distances for 3-21G gauche3-1,5-hexadiene optimisation:
Item Value Threshold Converged?
Maximum Force 0.000054 0.000450 YES
RMS Force 0.000014 0.000300 YES
Maximum Displacement 0.001584 0.001800 YES
RMS Displacement 0.000450 0.001200 YES
Predicted change in Energy=-5.323198D-08
Optimization completed.
-- Stationary point found.
Result of Symmeterise Function: C1
Total Energy Minimum Value for 3-21G optimisation of gauche3-1,5-hexadiene Molecule: -231.6926612 Hartee
Summary Table of Optimisations
The structures obtained from the first three optimisations were then compared with the provided structures in Appendix 1[1]. All structures were identified and there were little marked differences in energies.
| 1,5-Hexadiene Structure | Minimum Energy (Hartee) | Symmetry | Similar to Appendix 1 Structure (A1S) | Symmetry of A1S | Energy of A1S |
|---|---|---|---|---|---|
| Anti Linkage | -231.6926024 | C2 | Anti 1 | C2 | -231.69260 |
| Gauche Linkage | -231.691667 | C2 | Gauche 2 | C2 | -231.69167 |
| Gauche | -231.6926612 | C1 | Gauche 3 | C1 | -231.69266 |
Optimisation of anti2 1,5-hexadiene
The Ci anti2 conformation of 1,5-hexadiene was drawn and then optimised at the HF/3-21G level using 500MB %Mem.
Link to anti2 1,5-hexadiene HF/3-21G optimisation .LOG file: click here
test molecule |
Results summary table:
Table 5
| MS_15_hexadiene_anti2l_react | ||
| File Name | MS_15_HEXADIENE_ANTI2_REACT | |
| File Type | .log | |
| Calculation Type | FOPT | |
| Calculation Method | RHF | |
| Basis Set | 3-21G | |
| Charge | 0 | |
| Spin | Singlet | |
| E(RHF) | -231.6925353 | a.u. |
| RMS Gradient Norm | 0.00001711 | a.u. |
| Imaginary Freq | ||
| Dipole Moment | 0.0003 | Debye |
| Point Group | C1 | |
| Job cpu time: 2 minutes 42.0 seconds | ||
Calculation Log: 'Item' table of converged forces and distances for 3-21G anti 2 1,5-hexadiene optimisation:
Item Value Threshold Converged?
Maximum Force 0.000039 0.000450 YES
RMS Force 0.000008 0.000300 YES
Maximum Displacement 0.001323 0.001800 YES
RMS Displacement 0.000477 0.001200 YES
Predicted change in Energy=-2.270653D-08
Optimization completed.
-- Stationary point found.
Result of Symmeterise Function: Ci
Total Energy Minimum Value for HF/3-21G optimisation of anti 2 1,5-hexadiene Molecule: -231.6925353 Hartee
If we compare the minimum energy calculated from the HF/3-21G optimisation of anti 2 1,5-hexadiene (-231.6925353 Hartee)with that of the value in Appendix 1 (-231.69254 Hartee[1]) we immdeiately see that the eneries are very close indeed. However we can get an even closer value by carrying out a further optimisation with a higher level basis set. In this case the B3LYP/6-31G* set was used.
Further optimisation of anti2 1,5-hexadiene
The .chk file from the HF/3-21G optimisation of anti2 conformation of 1,5-hexadiene was used as the starting point for this optimisation. The structure was re-optimised at the DFT/B3LYP/6-31G* level using 500MB %Mem.
Link to anti2 1,5-hexadiene DFT/B3LYP/6-31G* optimisation .LOG file: click here
test molecule |
Results summary table:
Table 6
| MS_15_hexadiene_DFT_anti2_react | ||
| File Name | MS_15_HEXADIENE_DFT_ANTI2_REACT | |
| File Type | .log | |
| Calculation Type | FOPT | |
| Calculation Method | RB3LYP | |
| Basis Set | 6-31G(d) | |
| Charge | 0 | |
| Spin | Singlet | |
| E(RB3LYP) | -234.6117104 | a.u. |
| RMS Gradient Norm | 0.00001368 | a.u. |
| Imaginary Freq | ||
| Dipole Moment | 0 | Debye |
| Point Group | C1 | |
| Job cpu time: 5 minutes 18.0 seconds | ||
Calculation Log: 'Item' table of converged forces and distances for DFT/B3LYP/6-31G* anti 2 1,5-hexadiene optimisation:
Item Value Threshold Converged?
Maximum Force 0.000016 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.000264 0.001800 YES
RMS Displacement 0.000089 0.001200 YES
Predicted change in Energy=-1.687592D-08
Optimization completed.
-- Stationary point found.
Result of Symmeterise Function: Ci
Total Energy Minimum Value for DFT/B3LYP/6-31G* optimisation of anti 2 1,5-hexadiene Molecule: -234.6117104 Hartee
How much does the overall geometry change?
During the optimisation the central carbon-carbon bond rotates while the rest of the molecule stays relatively stationary. In addition to this the two double bonds at the ends of the molecule lengthen from 1.316 to 1.333 Å. If we also compare minimum energies we see that the energy has decreased from -231.6925353 to -234.6117104 Hartee which is quite a significant energy change on this scale.
Frequency Analysis of optimised anti2 1,5-hexadiene
A DFT/B3LYP/6-31G* frequency analysis was carried out on anti2 1,5-hexadiene using 500MB %Mem.
Link to anti 1,5-hexadiene DFT/B3LYP/6-31G* frequency analysis .LOG file: click here
Results Summary Table:
Table 7
| MS_anti_2_DFT_FREQ | ||
| File Name | MS_anti_2_DFT_FREQ | |
| File Type | .log | |
| Calculation Type | FREQ | |
| Calculation Method | RB3LYP | |
| Basis Set | 6-31G(d) | |
| Charge | 0 | |
| Spin | Singlet | |
| E(RB3LYP) | -234.6117104 | a.u. |
| RMS Gradient Norm | 0.00001367 | a.u. |
| Imaginary Freq | 0 | |
| Dipole Moment | 0 | Debye |
| Point Group | C1 | |
| Job cpu time: 5 minutes 37.0 seconds | ||
Calculation Log: 'Item' table of converged forces and distances for DFT/B3LYP/6-31G* anti 2 1,5-hexadiene frequency analysis:
Item Value Threshold Converged?
Maximum Force 0.000036 0.000450 YES
RMS Force 0.000014 0.000300 YES
Maximum Displacement 0.000290 0.001800 YES
RMS Displacement 0.000116 0.001200 YES
Predicted change in Energy=-1.604386D-08
Optimization completed.
-- Stationary point found.
Low Frequencies:
Low frequencies --- -9.4897 0.0009 0.0010 0.0011 3.7534 13.0192 Low frequencies --- 74.2855 80.9995 121.4166
Computed IR spectrum:
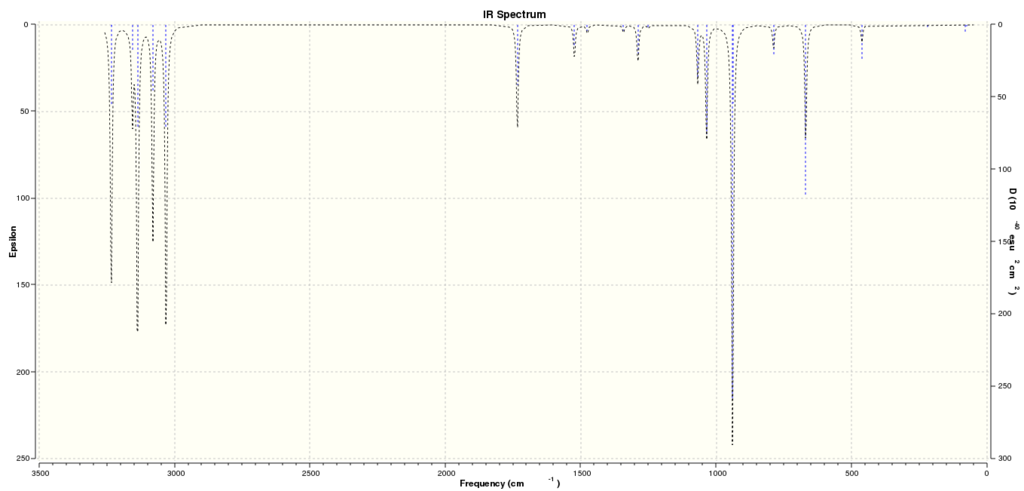
Results File: Thermochemistry
Sum of electronic and zero-point Energies = -234.469204 Hartee Sum of electronic and thermal Energies = -234.461857 Hartee Sum of electronic and thermal Enthalpies = -234.460913 Hartee Sum of electronic and thermal Free Energies = -234.500777 Hartee
Optimizing the "Chair" and "Boat" Transition Structures
Optimisation of Allyl Fragment
A HF/3-21G optimisation was carried out on the allyl fragment.
Link to allyl fragment HF/3-21G optimisation .LOG file: click here
test molecule |
Results Summary Table:
Table 8
| MS_15_hexadiene_allyl_react | ||
| File Name | MS_ALLYL_REACT | |
| File Type | .log | |
| Calculation Type | FOPT | |
| Calculation Method | UHF | |
| Basis Set | 3-21G | |
| Charge | 0 | |
| Spin | Doublet | |
| E(UHF) | -115.8230401 | a.u. |
| RMS Gradient Norm | 0.00002921 | a.u. |
| Imaginary Freq | ||
| Dipole Moment | 0.0291 | Debye |
| Point Group | C1 | |
| Job cpu time: 42.0 seconds | ||
Calculation Log: 'Item' table of converged forces and distances for HF/3-21G optimisation:
Item Value Threshold Converged?
Maximum Force 0.000088 0.000450 YES
RMS Force 0.000020 0.000300 YES
Maximum Displacement 0.000138 0.001800 YES
RMS Displacement 0.000047 0.001200 YES
Predicted change in Energy=-1.431856D-08
Optimization completed.
-- Stationary point found.
Total Energy Minimum Value for HF/3-21G optimisation of allyl fragment: -115.8230401 Hartee
Optimisation and Frequency Analysis of 'Chair' Transition State
A HF/3-21G opt+freq analysis was carried out on the 'chair' transition state.
Link to 'chair' transition state HF/3-21G opt+freq .LOG file: click here
test molecule |
Results Summary Table:
Table 9
| MS_chair_ts_guess_OPT_FREQ | ||
| File Name | MS_CHAIR_TS_GUESS_OPT_FREQ | |
| File Type | .log | |
| Calculation Type | FREQ | |
| Calculation Method | RHF | |
| Basis Set | 3-21G | |
| Charge | 0 | |
| Spin | Singlet | |
| E(RHF) | -231.6193224 | a.u. |
| RMS Gradient Norm | 0.00002606 | a.u. |
| Imaginary Freq | 1 | |
| Dipole Moment | 0.0004 | Debye |
| Point Group | C1 | |
| Job cpu time: 18.0 seconds | ||
Calculation Log: 'Item' table of converged forces and distances for HF/3-21G opt+freq:
Item Value Threshold Converged?
Maximum Force 0.000032 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.001108 0.001800 YES
RMS Displacement 0.000130 0.001200 YES
Predicted change in Energy=-4.599200D-08
Optimization completed.
-- Stationary point found.
Low Frequencies:
Low frequencies --- -817.9109 -0.0009 -0.0007 -0.0003 2.0191 3.7562 Low frequencies --- 5.0362 209.6163 395.9915
Imaginary Vibration at -817.91cm-1 corresponding to Cope rearrangement:
Imaginary Vibration |
Frozen Coordinates Method
The frozen coordinates method was used to perform a HF/3-21G transition state optimisation on the chair transition state while keeping the distance between the two ends of the allyl fragments fixed at 2.2Å. Following this a further HF/3-21G optimisation was carried out on the previously frozen bonds between the ends of the two allyl fragments.
Link to chair TS HF/3-21G frozen coordinate optimisation .LOG file: click here
Link to chair TS HF/3-21G terminal bonds optimisation .LOG file: click here
|
|
Results Summary Tables:
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Calculation Log: 'Item' table of converged forces and distances for HF/3-21G frozen coordinate optimisation:
Item Value Threshold Converged?
Maximum Force 0.000041 0.000450 YES
RMS Force 0.000013 0.000300 YES
Maximum Displacement 0.001652 0.001800 YES
RMS Displacement 0.000415 0.001200 YES
Predicted change in Energy=-6.910389D-07
Optimization completed.
-- Stationary point found.
Calculation Log: 'Item' table of converged forces and distances for HF/3-21G terminal bonds optimisation:
Item Value Threshold Converged?
Maximum Force 0.000028 0.000450 YES
RMS Force 0.000008 0.000300 YES
Maximum Displacement 0.001011 0.001800 YES
RMS Displacement 0.000184 0.001200 YES
Predicted change in Energy=-3.063197D-07
Optimization completed.
-- Stationary point found.
Total Energy Minimum Value for HF/3-21G frozen coordinate optimisation of chair TS: -231.6151844 Hartee
Total Energy Minimum Value for HF/3-21G terminal bonds optimisation of chair TS: -231.619322 Hartee
Optimisation of Boat TS
A QST2 HF/3-21G opt+freq analysis was carried out on the anti2 conformation of 1,5-hexadiene. The optimisation failed since Gaussian was unable to locate the boat transition state due to inability to rotate about the central bonds. As a result the chair transition structure was located however this structure was much more dissociated than the previously optimised chair transition state structure. It was clear that it would not be possible to locate the boat TS with the reactants and products in the anti2 conformation.
Link to failed TS HF/3-21G QST2 optimisation of anti2 1,5-hexadiene .LOG file: click here
test molecule |
Results Summary Table:
Table 12
| MS_boat_ts_OPT_FREQ_QST2_FAIL | ||
| File Name | MS_BOAT_TS_OPT_FREQ_QST2_FAIL | |
| File Type | .log | |
| Calculation Type | FREQ | |
| Calculation Method | RHF | |
| Basis Set | 3-21G | |
| Charge | 0 | |
| Spin | Singlet | |
| E(RHF) | -231.6193224 | a.u. |
| RMS Gradient Norm | 0.00003359 | a.u. |
| Imaginary Freq | 1 | |
| Dipole Moment | 0.0011 | Debye |
| Point Group | C1 | |
| Job cpu time: 1 minutes 31.0 seconds | ||
Calculation Log: 'Item' table of converged forces and distances for HF/3-21G QST2 opt+freq analysis:
Item Value Threshold Converged?
Maximum Force 0.000079 0.000450 YES
RMS Force 0.000020 0.000300 YES
Maximum Displacement 0.001539 0.001800 YES
RMS Displacement 0.000427 0.001200 YES
Predicted change in Energy=-1.124514D-07
Optimization completed.
-- Stationary point found.
Total Energy Minimum Value for HF/3-21G QST2 opt+freq analysis of chair TS: -231.6193224 Hartee
Following the failure of the previous optimisation the initial structure was altered to more resemble the boat transition structure. This was achieved by changing the central bond angle to 0o and then altering the adjacent angles to 100o so that the structure more resembled the gauche2 conformation in Appendix 1[1]. Another QST2 HF/3-21G opt+freq analysis was carried out on the altered structure of 1,5-hexadiene and resulted in the 'Boat'transition structure of 1,5-hexadiene.
Link to successful TS HF/3-21G QST2 optimisation of 1,5-hexadiene .LOG file: click here
test molecule |
Results Summary Table:
Table 13
| MS_15_hexadiene_boat_QST2_OPT_FREQ_react | ||
| File Name | MS_15_HEXADIENE_BOAT_QST2_OPT_FREQ_REACT | |
| File Type | .log | |
| Calculation Type | FREQ | |
| Calculation Method | RHF | |
| Basis Set | 3-21G | |
| Charge | 0 | |
| Spin | Singlet | |
| E(RHF) | -231.6028022 | a.u. |
| RMS Gradient Norm | 0.0000423 | a.u. |
| Imaginary Freq | 1 | |
| Dipole Moment | 0.1584 | Debye |
| Point Group | C1 | |
| Job cpu time: 15.0 seconds | ||
Calculation Log: 'Item' table of converged forces and distances for HF/3-21G QST2 opt+freq analysis:
Item Value Threshold Converged?
Maximum Force 0.000095 0.000450 YES
RMS Force 0.000025 0.000300 YES
Maximum Displacement 0.001687 0.001800 YES
RMS Displacement 0.000370 0.001200 YES
Predicted change in Energy=-2.536545D-07
Optimization completed.
-- Stationary point found.
Total Energy Minimum Value for HF/3-21G QST2 opt+freq analysis of boat TS: -231.6028022 Hartee
Intrinsic Reaction Coordinates Method
The Intrinsic Reaction Coordinates Method works by making a series of small changes in the geometry of the molecule so as to follow the steepest part of the reaction energy surface.
An initial optimisation was conducted specifying 50 steps along the IRC and computing the reaction coordinate in the forward direction. After 44 steps a minimum geometry was located which highly resembled the gauche2 structure in Appendix 1[1].
Link to IRC HF/3-21G calculation for chair TS .LOG file: click here
test molecule |
Results Summary Table:
Table 14
| MS_chair_ts_guess_IRC | ||
| File Name | MS_CHAIR_TS_GUESS_IRC | |
| File Type | .log | |
| Calculation Type | IRC | |
| Calculation Method | ||
| Basis Set | ||
| Charge | 0 | |
| Spin | Singlet | |
| E(RHF) | -231.6915792 | a.u. |
| RMS Gradient Norm | 0.00015224 | a.u. |
| Imaginary Freq | ||
| Dipole Moment | 0.3631 | Debye |
| Point Group | C1 | |
| Job cpu time: 10 minutes 0.0 seconds | ||
Total Energy Minimum Value for IRC calculation on chair ts: -231.6915792 Hartee
Further Optimisation of 'Chair' and 'Boat' Structures
Once the 'chair' and 'boat' transition structures had been located they were both further optimised at the B3LYP/6-31G* level.
Link to chair TS B3LYP/6-31G* optimisation .LOG file: click here
Link to boat TS B3LYP/6-31G* optimisation .LOG file: click here
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Calculation Log: 'Item' table of converged forces and distances for B3LYP/6-31G* optimisation of chair TS:
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000213 0.001800 YES
RMS Displacement 0.000066 0.001200 YES
Predicted change in Energy=-5.892603D-09
Optimization completed.
-- Stationary point found.
Calculation Log: 'Item' table of converged forces and distances for B3LYP/6-31G* optimisation of chair TS:
Item Value Threshold Converged?
Maximum Force 0.000237 0.000450 YES
RMS Force 0.000053 0.000300 YES
Maximum Displacement 0.001435 0.001800 YES
RMS Displacement 0.000476 0.001200 YES
Predicted change in Energy=-1.633179D-06
Optimization completed.
-- Stationary point found.
Total Energy Minimum Value for B3LYP/6-31G* chair TS: -232.893136 Hartee
Total Energy Minimum Value for B3LYP/6-31G* boat TS: -232.8783953 Hartee
Summary of Energies (Hartee)
Chair TS
HF/3-21G Optimisation:
Electronic Energy= -231.619322 Sum of electronic and zero-point Energies= -231.466702 Sum of electronic and thermal Energies= -231.461343
B3LYP/6-31G* Optimisation:
Electronic Energy= -234.543093 Sum of electronic and zero-point Energies= -234.414929 Sum of electronic and thermal Energies= -234.409008
Boat TS
HF/3-21G Optimisation:
Electronic Energy= -231.602802 Sum of electronic and zero-point Energies= -231.450930 Sum of electronic and thermal Energies= -231.445302
B3LYP/6-31G* Optimisation:
Electronic Energy= -234.543093 Sum of electronic and zero-point Energies= -234.402339 Sum of electronic and thermal Energies= -234.396005
Reactant (anti2)
HF/3-21G Optimisation:
Electronic Energy= -231.692535 Sum of electronic and zero-point Energies= -231.539545 Sum of electronic and thermal Energies= -231.532574
B3LYP/6-31G* Optimisation:
Electronic Energy= -234.611703 Sum of electronic and zero-point Energies= -234.469211 Sum of electronic and thermal Energies= -234.461856
Summary of activation energies (in kcal/mol)
*1 hartree = 627.509 kcal/mol
| HF/3-21G | HF/3-21G | B3LYP/6-31G* | B3LYP/6-31G* | Expt. | |
| at 0 K | at 298.15 K | at 0 K | at 298.15 K | at 0 K | |
| ΔE (Chair) | 45.71 | 44.70 | 34.06 | 33.16 | 33.5 ± 0.5 |
| ΔE (Boat) | 55.61 | 54.76 | 41.96 | 41.32 | 44.7 ± 2.0 |
Experimental values obtained from [1]
The table above summarises the activation energies calculated for the anti2 reactant and the Chair and Boat transition structures. From this it is clear that the chair transition state has the lower activation energy relative to the boat and thus is the preferred transition structure for the reaction. We can also see that the values calculated by Gaussian aren't that dissimilar from those obtained from experiment.
The Diels Alder Cycloaddition
Cis-Butadiene
Optimisaton
The AM1 semi-empirical molecular orbital method was used to optimise the structure of cis-butadiene.
Link to cis-butadiene AM1 semi-empirical optimisation .LOG file: click here
test molecule |
Results Summary Table:
| MS_cis_butadiene_AM1_OPT | ||
| File Name | MS_CIS_BUTADIENE_AM1_OPT | |
| File Type | .log | |
| Calculation Type | FOPT | |
| Calculation Method | RAM1 | |
| Basis Set | ZDO | |
| Charge | 0 | |
| Spin | Singlet | |
| E(RAM1) | 0.04879719 | a.u. |
| RMS Gradient Norm | 0.00001745 | a.u. |
| Imaginary Freq | ||
| Dipole Moment | 0.0414 | Debye |
| Point Group | C1 | |
| Job cpu time: 27.0 seconds | ||
Calculation Log: 'Item' table of converged forces and distances for AM1 semi-empirical optimisation of cis-butadiene:
Item Value Threshold Converged?
Maximum Force 0.000030 0.000450 YES
RMS Force 0.000011 0.000300 YES
Maximum Displacement 0.000388 0.001800 YES
RMS Displacement 0.000162 0.001200 YES
Predicted change in Energy=-9.691070D-09
Optimization completed.
-- Stationary point found.
HOMO/LUMO of cis-butadiene
The molecular orbitals of cis-butadiene were computed and can be seen below:
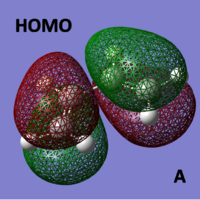 |
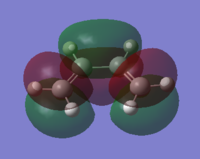
|
As you can see from above, the LUMO of cis-butadiene is symmetric with respect to a plane and anti-symmetric with respect to rotation.
The HOMO of cis-butadiene is the opposite being anti-symmetric with respect to a plane and symmetric with respect to rotation.
Ethylene + cis-butadiene transition structure
Optimisation
An AM1 semi-empirical molecular orbital method was used to optimise the transition structure of cis-btadiene and ethylene.
Link to cis-butadiene AM1 semi-empirical optimisation .LOG file: click here
test molecule |
Results Summary Table:
| MS_DA_TS_AM1_OPT | ||
| File Name | MS_DA_TS_AM1_OPT | |
| File Type | .log | |
| Calculation Type | FTS | |
| Calculation Method | RAM1 | |
| Basis Set | ZDO | |
| Charge | 0 | |
| Spin | Singlet | |
| E(RAM1) | 0.11165465 | a.u. |
| RMS Gradient Norm | 0.00000465 | a.u. |
| Imaginary Freq | ||
| Dipole Moment | 0.5605 | Debye |
| Point Group | C1 | |
| Job cpu time: 55.0 seconds | ||
Calculation Log: 'Item' table of converged forces and distances for AM1 semi-empirical optimisation of cis-butadiene and ethylene TS:
Item Value Threshold Converged?
Maximum Force 0.000009 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000116 0.001800 YES
RMS Displacement 0.000025 0.001200 YES
Predicted change in Energy=-2.066231D-10
Optimization completed.
-- Stationary point found.
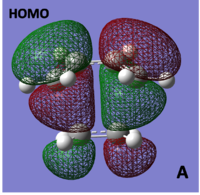
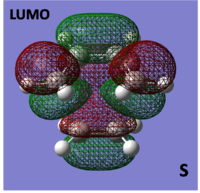
HOMO/LUMO of cis-butadiene and Ethylene TS
The molecular orbitals of the transition structure were computed and can be seen below:
 |
|
When looking at the molecular orbitals calculated by Gaussian for the transition state it is seen that the HOMO of the TS is anti-symmetric with respect to the plane and the LUMO is symmetric with respect to the plane.
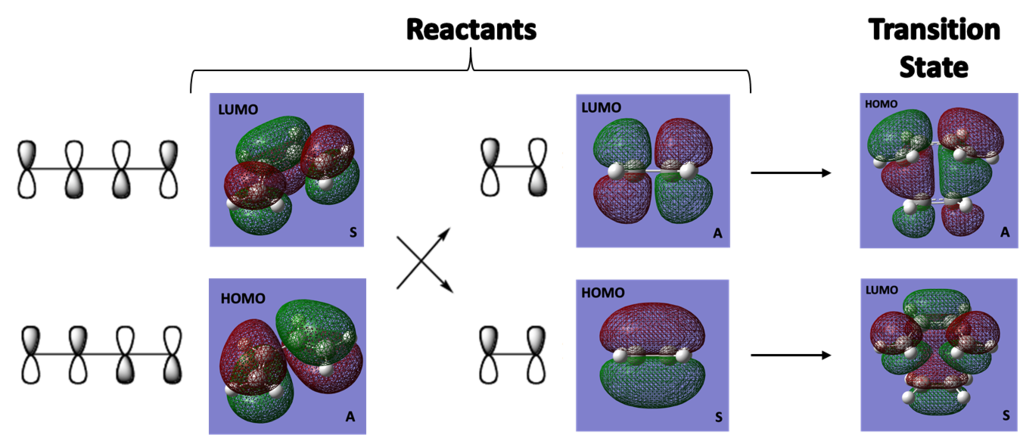
Why is the reaction formally 'allowed?'
We can predict whether a reaction will be allowed or disallowed by inspecting the orbitals of the reactants. If the orbital symmetry of the reacting molecules is conserved, then the reaction is formally allowed. If orbital symmetry is not conserved then the reaction is formally disallowed. As you can see in the diagram below [2], in this particular reaction orbital symmetry is conserved and thus the reaction is formally allowed.
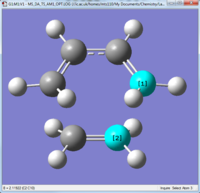
Geometry of the transition structure
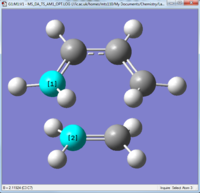 |
|
As you can see the transition structure was Cs symmetry and the partially formed bonds are 2.12Å in length.
| C-C Bond Lengths (Å) | |
| Sp2 - Sp2 | 1.34Å [3] |
| Sp3 - Sp3 | 1.54Å [3] |
| VDW Radius(C) | 1.70Å [4] |
From the above values we can see that if we double the VDW radius of carbon (3.40Å), the calculated TS distance between the two carbon atoms (2.12Å) is less than this value. This tells us that there is overlap between the orbitals on the carbon atoms and is an indication that the product bonds are beginning to form. If we now consider the values of the Sp2-Sp2 and Sp3-Sp3 carbon-carbon distances we see that this is less than the transition state value. This can be understood when we consider that the bonds between the two atoms have not fully formed yet.
Transition State Vibrations
After conducting a frequency analysis of the transition state an imaginary vibration was observed at -956.30cm-1. From the animation below it can be seen clearly that this vibration corresponds to the Diels Alder Bond Formation:
Vibration |
Cyclohexa-1,3-diene reaction with maleic anhydride
Locating the Exo Transition Structure
To locate the transition structures in the Diels Alder reaction between Cyclohexa-1,3-diene and maleic anhydride the frozen coordinates method used in the previous question was utilised. This method involved specifying certain bonds in the structure to stay 'frozen' throughout the optimisation, then once the optimisation was complete these bonds were unfrozen and re-optimised using a transition state optimisation method.
Optimisation
To begin with, the exo structure of the product between Cyclohexa-1,3-diene and maleic anhydride was drawn. This structure was then altered to more resemble the transition state and the bonds that form between the two reactant molecules during the reaction were lengthened to 2.2Å. A HF/321G optimisation was carried out upon the structure ensuring that it was fully optimised, then subsequently the structure was unfrozen and a HF/321G TS(Berry) optimisation was carried out.
Link to TS frozen/unfrozen optimisations on D-Space: Frozen:DOI:10042/21478 Unfrozen:DOI:10042/21479
Link to TS HF/3-21G frozen coordinate optimisation .LOG file: click here
Link to TS HF/3-21G terminal bonds optimisation .LOG file: click here
Results Summary Tables:
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Calculation Log: 'Item' table of converged forces and distances for HF/3-21G frozen coordinate optimisation:
Item Value Threshold Converged?
Maximum Force 0.000023 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000960 0.001800 YES
RMS Displacement 0.000223 0.001200 YES
Predicted change in Energy=-1.298578D-08
Optimization completed.
-- Stationary point found.
Calculation Log: 'Item' table of converged forces and distances for HF/3-21G terminal bonds optimisation:
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000320 0.001800 YES
RMS Displacement 0.000045 0.001200 YES
Predicted change in Energy=-2.065548D-09
Optimization completed.
-- Stationary point found.
Total Energy Minimum Value for HF/3-21G frozen coordinate optimisation of TS: -605.605355 Hartee
Total Energy Minimum Value for HF/3-21G terminal bonds optimisation of TS: -605.6035913 Hartee
Further Optimisation
Subsequently a higher level calculation was run at the B3YLP/631G* level of theory. The results were as follows:
Link to TS frozen/unfrozen B3YLP/631G* optimisations on D-Space: Frozen:DOI:10042/21496 Unfrozen:DOI:10042/21497
Link to TS B3YLP/631G* frozen coordinate optimisation .LOG file: click here
Link to TS B3YLP/631G* terminal bonds optimisation .LOG file: click here
|
|
Results Summary Tables:
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Calculation Log: 'Item' table of converged forces and distances for B3YLP/631G* frozen coordinate optimisation:
Item Value Threshold Converged?
Maximum Force 0.000101 0.000450 YES
RMS Force 0.000012 0.000300 YES
Maximum Displacement 0.001383 0.001800 YES
RMS Displacement 0.000312 0.001200 YES
Predicted change in Energy=-2.231145D-07
Optimization completed.
-- Stationary point found.
Calculation Log: 'Item' table of converged forces and distances for B3YLP/631G* terminal bonds optimisation:
Item Value Threshold Converged?
Maximum Force 0.000014 0.000450 YES
RMS Force 0.000002 0.000300 YES
Maximum Displacement 0.001020 0.001800 YES
RMS Displacement 0.000184 0.001200 YES
Predicted change in Energy=-2.535191D-08
Optimization completed.
-- Stationary point found.
Total Energy Minimum Value for B3YLP/631G* frozen coordinate optimisation of TS: -612.6810343 Hartee
Total Energy Minimum Value for B3YLP/631G* terminal bonds optimisation of TS: -612.6793109 Hartee
Frequency Analysis
Following the higher level optimisation of the Exo transition state, a frequency analysis was conducted to ensure that the imaginary vibration observed corresponded to the formation of the bonds between reactants and product.
Link to Frequency Analysis of Exo Transition Structure on D-Space: DOI:10042/21498
Link to Frequency Analysis .LOG file: click here
Low Frequencies:
Low frequencies --- -448.6262 -13.8843 -11.7111 -0.0010 -0.0007 0.0003 Low frequencies --- 3.2147 53.3274 109.1042 ****** 1 imaginary frequencies (negative Signs) ******
After conducting the frequency analysis of the transition state an imaginary vibration was observed at -448.63cm-1. From the animation below it can be seen clearly that this vibration corresponds to the Diels Alder Bond Formation:
Vibration |
HOMO/LUMO of Cyclohexa-1,3-diene reaction with maleic anhydride TS
The molecular orbitals of the Exo transition structure were computed and can be seen below:
 |
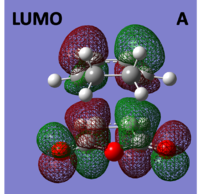
|
When looking at the molecular orbitals calculated by Gaussian for the Exo transition state it is seen that the HOMO of the TS is anti-symmetric with respect to the plane and the LUMO is also anti-symmetric with respect to the plane.
Locating the Endo Transition Structure
To locate the transition structures in the Diels Alder reaction between Cyclohexa-1,3-diene and maleic anhydride the frozen coordinates method used in the previous question was utilised. This method involved specifying certain bonds in the structure to stay 'frozen' throughout the optimisation, then once the optimisation was complete these bonds were unfrozen and re-optimised using a transition state optimisation method.
Optimisation
To begin with, the endo structure of the product between Cyclohexa-1,3-diene and maleic anhydride was drawn. This structure was then altered to more resemble the transition state and the bonds that form between the two reactant molecules during the reaction were lengthened to 2.2Å. A HF/321G optimisation was carried out upon the structure ensuring that it was fully optimised, then subsequently the structure was unfrozen and a HF/321G TS(Berry) optimisation was carried out.
Link to TS frozen/unfrozen optimisations on D-Space: Frozen:DOI:10042/21499 Unfrozen:DOI:10042/21500
Link to TS HF/3-21G frozen coordinate optimisation .LOG file: click here
Link to TS HF/3-21G terminal bonds optimisation .LOG file: click here
Results Summary Tables:
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Calculation Log: 'Item' table of converged forces and distances for HF/3-21G frozen coordinate optimisation:
Item Value Threshold Converged?
Maximum Force 0.000101 0.000450 YES
RMS Force 0.000017 0.000300 YES
Maximum Displacement 0.001225 0.001800 YES
RMS Displacement 0.000243 0.001200 YES
Predicted change in Energy=-1.492299D-07
Optimization completed.
-- Stationary point found.
Calculation Log: 'Item' table of converged forces and distances for HF/3-21G terminal bonds optimisation:
Item Value Threshold Converged?
Maximum Force 0.000042 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000585 0.001800 YES
RMS Displacement 0.000097 0.001200 YES
Predicted change in Energy= 7.269361D-08
Optimization completed.
-- Stationary point found.
Total Energy Minimum Value for HF/3-21G frozen coordinate optimisation of TS: -605.6107979 Hartee
Total Energy Minimum Value for HF/3-21G terminal bonds optimisation of TS: -605.6103683 Hartee
Further Optimisation
Subsequently a higher level calculation was run at the B3YLP/631G* level of theory. The results were as follows:
Link to Endo TS frozen/unfrozen B3YLP/631G* optimisations on D-Space: Frozen:DOI:10042/21502 Unfrozen:DOI:10042/21503
Link to Endo TS B3YLP/631G* frozen coordinate optimisation .LOG file: click here
Link to Endo TS B3YLP/631G* terminal bonds optimisation .LOG file: click here
|
|
Results Summary Tables:
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Calculation Log: 'Item' table of converged forces and distances for B3YLP/631G* frozen coordinate optimisation:
Item Value Threshold Converged?
Maximum Force 0.000309 0.000450 YES
RMS Force 0.000034 0.000300 YES
Maximum Displacement 0.001025 0.001800 YES
RMS Displacement 0.000278 0.001200 YES
Predicted change in Energy=-5.845744D-07
Optimization completed.
-- Stationary point found.
Calculation Log: 'Item' table of converged forces and distances for B3YLP/631G* terminal bonds optimisation:
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000173 0.001800 YES
RMS Displacement 0.000026 0.001200 YES
Predicted change in Energy=-5.951088D-10
Optimization completed.
-- Stationary point found.
Total Energy Minimum Value for B3YLP/631G* frozen coordinate optimisation of TS: -612.6843615 Hartee
Total Energy Minimum Value for B3YLP/631G* terminal bonds optimisation of TS: -612.6833968 Hartee
Frequency Analysis
Following the higher level optimisation of the Endo transition state, a frequency analysis was conducted to ensure that the imaginary vibration observed corresponded to the formation of the bonds between reactants and product.
Link to Frequency Analysis of Endo Transition Structure on D-Space: DOI:10042/21504
Link to Frequency Analysis .LOG file: click here
Low Frequencies:
Low frequencies --- -447.1212 -14.2253 0.0009 0.0010 0.0013 4.5293 Low frequencies --- 11.3191 59.6562 118.3681 ****** 1 imaginary frequencies (negative Signs) ******
After conducting the frequency analysis of the transition state an imaginary vibration was observed at -448.63cm-1. From the animation below it can be seen clearly that this vibration corresponds to the Diels Alder Bond Formation:
Vibration |
HOMO/LUMO of Cyclohexa-1,3-diene reaction with maleic anhydride TS
The molecular orbitals of the Endo transition structure were computed and can be seen below:
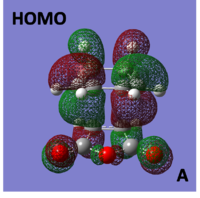 |
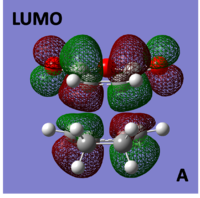
|
When looking at the molecular orbitals calculated by Gaussian for the Endo transition state it is seen that the HOMO of the TS is anti-symmetric with respect to the plane and the LUMO is also anti-symmetric with respect to the plane.
Relative Energies
The relative energies of the Exo and Endo transition structures can be seen in the table below:
Energies in Hartee
| HF/3-21G | B3LYP/6-31G* | |||
| Exo | -605.6035913 | -612.6793109 | ||
| Endo | -605.6103683 | -612.6833968 | ||
Immediately we can see there is an energy difference between the Exo and Endo structures, with the Endo structure being more stable by ~0.004 Hartee = ~2.56 kcal/mol. The question we must now ask is why is the Endo Form lower in energy? We can understand the reason for this if we consider the interactions between the orbitals in the transition state, a so called 'Secondary Orbital Overlap'. This will be discussed in the following section.
Discussion
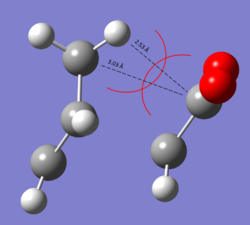
Before considering orbital interactions we will look at the bond lengths in the Endo and Exo structures so that we can determine whether there are any differences between the Endo and Exo forms which would indicate any steric strain there may be on the molecule.
If we consider the through space distance from the carbon and hydrogen atoms on the -CH2-CH2- fragment in the Exo form, we see that there is reasonable steric repulsion to disfavour this transition state (see diagram to right). This tells us that the Endo form is thus the kinetically more stable transition state since there are no steric repulsion's in the transition structure which would disfavour it.
If we now consider orbital interactions, it is clear from the Molecular Orbital diagrams of the Exo and Endo structures that there is the possibility of Orbital overlap in the Endo Structure. The pi orbitals in the C-O oxygen atoms can overlap with the C=C pi orbitals in HOMO structure of the Endo TS. This can be seen more clearly in the following diagram:
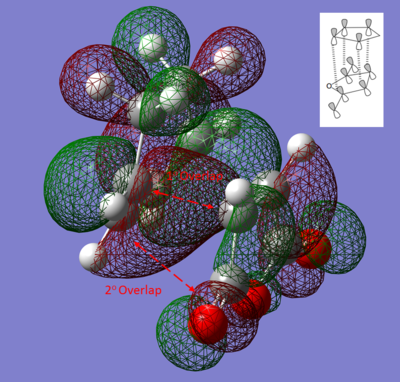
Black and white diagram obtained from [5]
If an optimisation were carried out on the Exo and Endo products from the Diels Alder reaction, it can be seen that the Exo product is lower in energy than the Endo. However if we were to actually carry out the reaction we would observe high Endo selectivity. This can now be explained by considering the lack of steric repulsion and secondary orbital overlap present in the Endo form if we ignore solvent effects and effects that are neglected by Guassian.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Mike Bearpark, Imperial College Chemistry Department. Module 3 - Optimising the Reactants and Products. [Online]. Available from: https://wiki.ch.ic.ac.uk/wiki/index.php?title=Mod:phys3#Optimizing_the_Reactants_and_Products [Accessed 2nd November 2012]
- ↑ Uniersity of Lethbridge Chemistry Department. Exercise 83 - Orbital symmetry cycloaddn. [Online]. Available from: http://classes.uleth.ca/201201/chem2600a/Exercise%2083A%20-%20Orbital%20symmetry%20cycloaddn.pdf [Accessed 1st November 2012]
- ↑ 3.0 3.1 Massachusetts Institute of Technology. Handout #3, 5.12 Spring 2003, 2/12/03 Physical Properties: Bond Length, Bond Strength & Acidity. [Online]. Available from: http://ocw.mit.edu/courses/chemistry/5-12-organic-chemistry-i-spring-2003/lecture-handouts/04.pdf [Accessed 30th October 2012]
- ↑ The Cambridge Crystallographic Data Centre. Element data and radii. [Online]. Available from: http://www.ccdc.cam.ac.uk/products/csd/radii/table.php4 [Accessed 1st November 2012]
- ↑ University of Michigan-Dearborn Chemistry Department. Secondary Orbital Overlap in the Transition State of the Diels-Alder Reaction. [Online]. Available from: http://www.umd.umich.edu/casl/natsci/chem/decamp/c226/Secondary%20Orbital%20Overlap.pdf [Accessed 2nd November 2012]