Rep:Mod:st4215
In this computational chemistry lab, several molecules were analysed using the programme GaussView, including NH3, H2, N2, and BH3. This allowed us to obtain important thermodynamic information regarding these molecules, such as the energy and structure of their most stable conformers as well as the shapes and energies of their molecular orbitals.
NH3
Molecule information
Summary
| NH3 Data | ||||
|---|---|---|---|---|
| ||||
| More info | ||||
| Name | Ammonia | |||
| Calculation method | RB3LYP | |||
| Basis set | 6-31G(d,p) | |||
| Final energy E(RB3LYP) | -56.55776873 a.u. | |||
| RMS Gradient Norm | 0.00000485 a.u. | |||
| Point group | C3V | |||
| N-H bond distance | 1.01798 Å | |||
| H-N-H bond angle | 105.741° | |||
Confirming successful optimisation
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
The optimisation file is linked here.
Vibrations
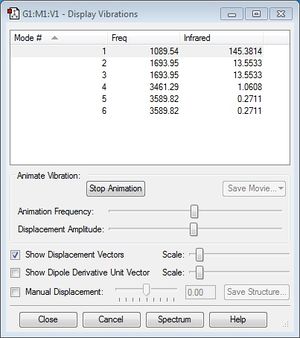
Using the 3N-6 rule, NH3 is expected to have 3(4)-6=6 vibrational modes. This is confirmed from the results of the Gaussian output, as seen from the screenshot shown below. We can also observe that vibrational modes 2 and 3 as well as 5 and 6 are degenerate, as each pair has the same frequency and therefore the same energy. Modes 1, 2 and 3 are "bending" vibrations while modes 4, 5 and 6 are "bond stretch" vibrations. Mode 4 is highly symmetric, and mode 1 is known as the "umbrella" mode.
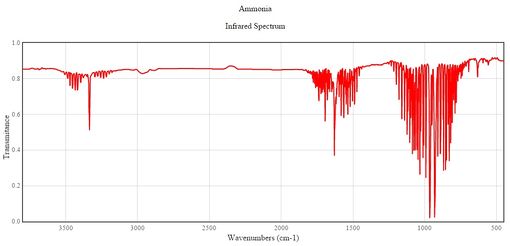
Based on this data, we can also expect to see 3 bands in an experimental infrared spectrum of gaseous ammonia. Although there are 6 vibrational modes, we would only expect to see 3 IR peaks corresponding to vibrational modes 1-4 (modes 2 and 3 give identical peaks), as the peaks of modes 5 and 6 are too weak to be observed. This is confirmed by literature IR spectrum data of ammonia, as seen below:
Atomic charge
Since N is more electronegative than H, we would expect N to have a negative charge while H to have a positive charge. This is confirmed by using GaussView, which shows that the atomic charge on N is -1.125 while that on H is 0.375.

Reactivity of NH3: The Haber Process
The Haber-Bosch process is the main industrial process by which nitrogen gas and hydrogen gas are converted to ammonia. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by the following reaction with H2, using an iron catalyst at high temperatures and pressures:
More information can be found here.
H2
Summary
| H2 Data | |||
|---|---|---|---|
|
| |||
| Summary | |||
| Name | Hydrogen | ||
| Calculation method | RB3LYP | ||
| Basis set | 6-31G(d,p) | ||
| Final energy E(RB3LYP) | -1.17853936 a.u. | ||
| RMS Gradient Norm | 0.00000017 a.u. | ||
| Point group | D∞h | ||
| H-H bond distance | 0.74279 Å | ||
Confirming successful optimisation
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
The optimisation file is linked here.
Vibrations
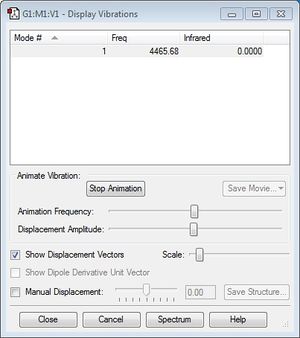
As can be seen in the screenshot below, the H2 molecule only has 1 frequency of vibration.
Atomic charge
Since the H2 molecule is diatomic with both atoms having the same electronegativity, the relative charge on each atom is 0 as shown below.

N2
Summary
| N2 Data | |||
|---|---|---|---|
|
| |||
| More info | |||
| Name | Nitrogen | ||
| Calculation method | RB3LYP | ||
| Basis set | 6-31G(d,p) | ||
| Final energy E(RB3LYP) | -109.52412868 a.u. | ||
| RMS Gradient Norm | 0.00000060 a.u. | ||
| Point group | D∞h | ||
| N-N bond distance | 1.10550 Å | ||
Confirming successful optimisation
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
The optimisation file is linked here.
Vibrations
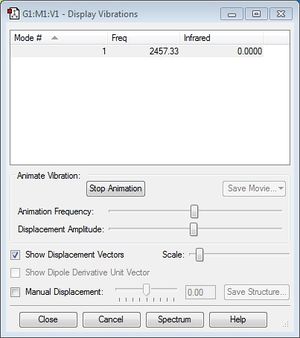
As can be seen in the screenshot below, the N2 molecule only has 1 frequency of vibration.
Atomic charge
Since the N2 molecule is diatomic with both atoms having the same electronegativity, the relative charge on each atom is 0 as shown below.

Energy of the Haber Process
The energy of the reaction can be computed as shown in the table below:
| Energy / a.u. | Energy / kJmol-1 | |
|---|---|---|
| E(NH3) | -56.55776873 | -148492.42 |
| 2*E(NH3) | -113.11553746 | -296984.84 |
| E(N2) | -109.52412868 | -287555.60 |
| E(H2) | -1.17853936 | -3094.255 |
| 3*E(H2) | -3.53561808 | -9282.77 |
| ΔE = 2*E(NH3) - [E(N2) + 3*E(H2)] | -0.0557907 | -146.48 |
The calculations above show that the energy for converting hydrogen and nitrogen into ammonia gas via the Haber process is -146.48 kJmol-1, to 2 decimal places. Since the reaction is exothermic, the product NH3 must be more stable than the reactants N2 and H2.
BH3
Molecule information
Summary
| BH3 Data | ||||
|---|---|---|---|---|
| ||||
| More info | ||||
| Name | Borane | |||
| Calculation method | RB3LYP | |||
| Basis set | 6-31G(d,p) | |||
| Final energy E(RB3LYP) | -26.61532364 a.u. | |||
| RMS Gradient Norm | 0.00000211 a.u. | |||
| Point group | D3h | |||
| B-H bond distance | 1.19232 Å | |||
| H-B-H bond angle | 120° | |||
Confirming successful optimisation
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000017 0.001800 YES RMS Displacement 0.000011 0.001200 YES
The optimisation file is linked here.
Vibrations
Similar to NH3, BH3 is expected to have 3(4)-6=6 vibrational modes using the 3N-6 rule. This is confirmed from the results of the Gaussian output, as seen from the screenshot shown below. We can also observe that vibrational modes 2 and 3 as well as 5 and 6 are degenerate, as each pair vibrates at the same frequency and therefore has the same energy. It can also be seen that modes 1, 2, 3 are "bending" vibrations while modes 4, 5 and 6 are "bond stretch" vibrations. Mode 4 in particular is highly symmetric.
Based on this data, we can also expect to see 4 bands in an experimental infrared spectrum of gaseous borane (since modes 2 and 3 as well as 5 and 6 give identical peaks).

Atomic charge
Since B is slightly less electronegative than H, we would expect B to have a slight positive charge while H to have a slight negative charge. This is confirmed by using GaussView, which shows that the atomic charge on B is 0.297 while that on H is -0.099.

Molecular orbitals
Selected molecular orbitals of BH3 are shown and explained below.





