Rep:Mod:ssv15
BH3
B3LYP/ 6-31G (d,p)


Completed BH3 frequency log file link: Media:SSV15_BH3_FREQ_631G.LOG
Low frequencies --- -0.2456 -0.1129 -0.0055 44.0270 45.1846 45.1853 Low frequencies --- 1163.6049 1213.5924 1213.5951
The frequencies observed above are unusually high, however after checking the frequency analysis lined up with the optimised BH3 molecule in terms of energy and whether the calculation converged, it was concluded that this due to inaccuracy of the software and computers running these simulations. This was also checked and confirmed by a demonstrator.
ssv15 BH3 optimised molecule |
| Wavenumber (cm-1) | Intensity (arbitrary units) | Symmetry | IR active? | Type | |
|---|---|---|---|---|---|
| 1164 | 92 | A2" | yes | bend | |
| 1214 | 14 | E' | slightly | bend | |
| 1214 | 14 | E' | slightly | bend | |
| 2580 | 0 | A1' | no | symmetric stretch | |
| 2713 | 126 | E' | yes | asymmetric stretch | |
| 2713 | 126 | E' | yes | asymmetric stretch |
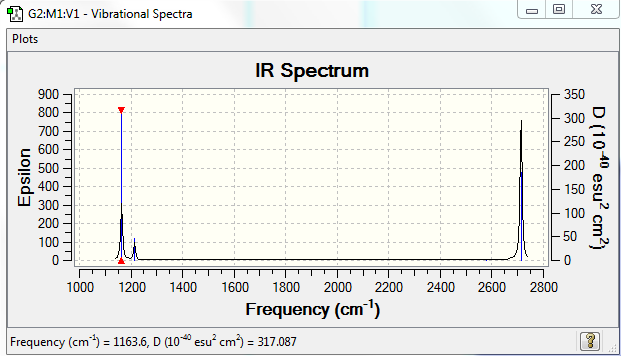
In the IR spectrum above there are 3 distinct peaks shows despite there being 6 vibrational modes. The vibration at wavenumber (2580 cm-1) is IR inactive due to there being no change in dipole moment during the symmetric stretch. There are also 2 degenerate pairs of vibrations, one set at 1214 cm-1 and the other at 2713 cm-1, therefore we only observe 2 peaks for these 4 vibrational modes. This leaves one mode at 1164 cm-1 which produces its own peak. In total we witness 3 peaks in the IR spectrum.
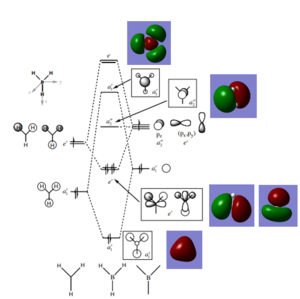
The above diagram shows there are no significant differences between the LCAO MO's and the real ones, therefore one can accurately predict the shapes of MO's using qualitative MO theory.
Ng611 (talk) 14:03, 6 June 2018 (BST) Good! There are some minor differences though that should be noted.
NH3
B3LYP/ 6-31G (d,p)

Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000012 0.001800 YES
RMS Displacement 0.000008 0.001200 YES
Predicted change in Energy=-9.844515D-11
Optimization completed.
-- Stationary point found.
Frequency NH3 log file: Media:SSV15_NH3_FREQ_631G.LOG
Low frequencies --- -0.0138 -0.0032 -0.0015 7.0783 8.0932 8.0937 Low frequencies --- 1089.3840 1693.9368 1693.9368
ssv15 NH3 optimised molecule |
BH3NH3
B3LYP/ 6-31G (d,p)
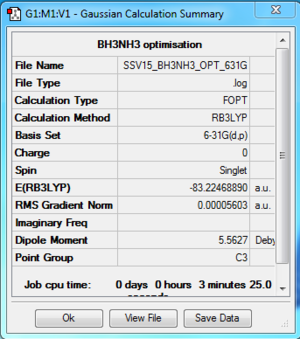
Item Value Threshold Converged?
Maximum Force 0.000164 0.000450 YES
RMS Force 0.000035 0.000300 YES
Maximum Displacement 0.000780 0.001800 YES
RMS Displacement 0.000338 0.001200 YES
Predicted change in Energy=-1.113039D-07
Optimization completed.
-- Stationary point found.
Frequency BH3NH3 log file Media:SSV15_BH3NH3_FREQ_631G.LOG
Low frequencies --- -19.3181 -0.0151 -0.0035 0.0170 9.1260 9.1294 Low frequencies --- 262.5021 631.2247 637.8482
ssv15 BH3NH3 optimised molecule |
BH3NH3 association energies
E(BH3) = -26.61532 au (5 d.p.) E(NH3) = -56.55777 au (5 d.p.) E(BH3NH3) = -83.22469 au (5 d.p.)
ΔE = E(BH3NH3) - [E(NH3) + E(BH3)] ΔE = -0.0516 au = 135 kJ/mol
Based on the information above the B-N dative bond is weak. Comparing it to the dissociation energy of C-H (337.2 kJmol[2]), a known strong bond, B-H has a much lower dissociation energy (135 kJ/mol).
Ng611 (talk) 14:04, 6 June 2018 (BST) Remember to cite your bond values (ideally from a textbook, databook, or paper).
Ng611 (talk) 14:05, 6 June 2018 (BST) Correct calculation and good consideration is given to the accuracy of the reported result.
BBr3
B3LYP/ 6-31G (d,p)
Ng611 (talk) 14:15, 6 June 2018 (BST) You presumably also used LANL2DZ for this calculation too and you should mention it with the rest of your job data here.
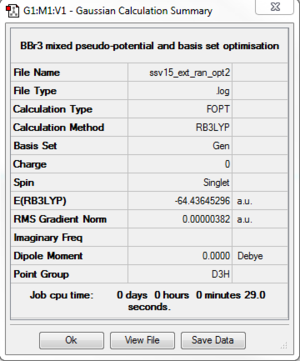
Item Value Threshold Converged?
Maximum Force 0.000008 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000036 0.001800 YES
RMS Displacement 0.000023 0.001200 YES
Predicted change in Energy=-4.027061D-10
Optimization completed.
-- Stationary point found.
Frequency BBr3 log file: Media:Ssv15_bbr3_freq.log
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
ssv15 BBr3 optimised molecule |
DSpace link: http://hdl.handle.net/10042/202459
Aromaticity
Benzene
B3LYP/ 6-31G (d,p)
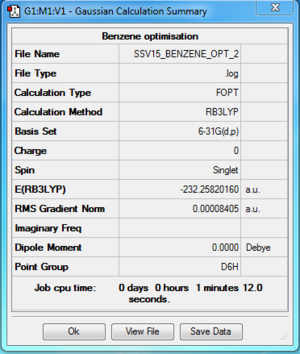
Item Value Threshold Converged?
Maximum Force 0.000194 0.000450 YES
RMS Force 0.000077 0.000300 YES
Maximum Displacement 0.000824 0.001800 YES
RMS Displacement 0.000289 0.001200 YES
Predicted change in Energy=-4.246203D-07
Optimization completed.
-- Stationary point found.
Completed benzene frequency log file link: Media: SSV15_BENZENE_FREQ_AND_MOS2.LOG
Low frequencies --- -2.1456 -2.1456 -0.0088 -0.0042 -0.0041 10.4835 Low frequencies --- 413.9768 413.9768 621.1390
ssv15 benzene optimised molecule |
Borazine
B3LYP/ 6-31G (d,p)
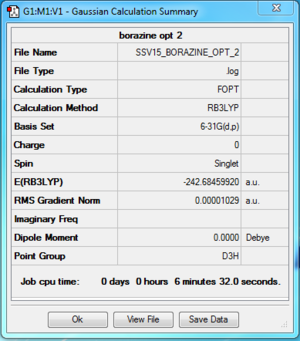
Item Value Threshold Converged?
Maximum Force 0.000011 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000064 0.001800 YES
RMS Displacement 0.000021 0.001200 YES
Predicted change in Energy=-3.183408D-09
Optimization completed.
-- Stationary point found.
Completed borazine frequency log file link: Media: SSV15_BORAZINE_FREQ_AND_MOS.LOG
Low frequencies --- -9.5469 -0.0004 0.0007 0.0010 4.2777 11.8176 Low frequencies --- 288.5478 290.5350 404.2742
ssv15 borazine optimised molecule |
NBO charge distribution analysis
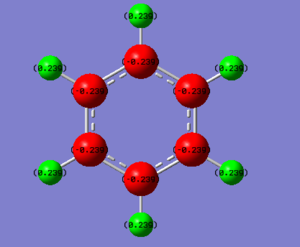
| Atom | Charge value | |
|---|---|---|
| Carbon | -0.239 | |
| Hydrogen | 0.239 |

Ng611 (talk) 14:06, 6 June 2018 (BST) Remember to use the same colour scale for both benzene and borazine, for ease of visual comparison. For reference, Benzene's charge should be very dark in colour.
| Atom | Charge value | |
|---|---|---|
| Nitrogen | -1.102 | |
| Boron | 0.747 | |
| Hydrogen (N-H) | 0.432 | |
| Hydrogen (B-H) | -0.077 |
From the data above, it can be seen that borazine has a more complex charge distribution than benzene. Benzene is a highly symmetric structure where majority of the charge is located in the delocalised pi ring. The carbon atoms in the ring have a charge of -0.239 whereas the terminal hydrogens have a more positive charge of 0.239. In borazine, the nitrogen atoms have the greatest electronegativity and so hold the most negative charge of -1.102 therefore implying the highest electron density is found at the nitrogen atoms in the molecule. The hydrogens attached to the nitrogen have the most postive charge of 0.432 due to the polarised bond that forms between them, due to the difference in electronegativity. Hydrogen is slightly more electronegative than boron, therefore the hydrogens attached to boron hold a slight negative charge and the borons a positive charge of 0.747.
Ng611 (talk) 14:08, 6 June 2018 (BST) Good discussion of the effects of electronegativity on the overall charge distribution. What do the partial charges sum to, and is there any difference in partial charge for atoms related by symmetry? You give a brief mention of symmetry in this section, but more detail is needed.
Comparable MO's of Benzene and Borazine

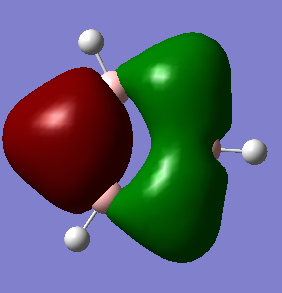
MO 9 of benzene and borazine are the lowest in energy that has been picked to compare. Benzene is a highly symmetric molecule causing an even split of phase and one nodal plane running through the centre of the molecule. Borazine has a much lower degree of symmetry due to the alternating boron and nitrogen atoms around the ring. The electronegative nitrogen atoms pull in electron density, therefore distorting the two phases present in MO 9 of borazine. There is also limited contribution from some terminal hydrogens.
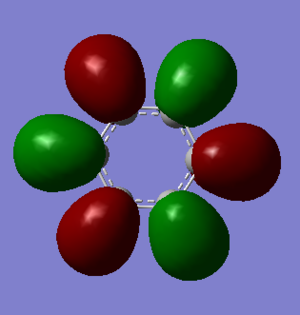
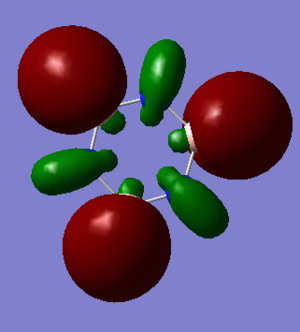
Antibonding character is displayed by MO's 13 and 16 between orbitals. The orbitals form around individual atoms in the ring structure forming 6 orbitals in both borazine and benzene. In benzene these orbitals are symmetric and evenly sized as benzene has an even charge distribution. As we know from the MO diagram, boron is less electronegative than hydrogen and so the boron orbitals lie higher in energy and therefore contribute more character to the antibonding orbitals. Therefore we see larger antibonding orbitals around the boron atoms in MO 16 of borazine.
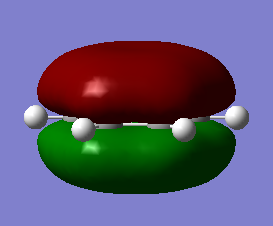

Both MO 17's of benzene and borazine display the pi orbitals above and below the delocalised ring structure, formed from the filled Pz orbitals on each atom in the ring structure interacting with one another. Both molecules display the same symmetry in these pi clouds despite having different symmetries otherwise.
Ng611 (talk) 14:13, 6 June 2018 (BST) Some good points made in this MO analysis, although they're somewhat inconsistently applied. As a minimum: for every orbital you compare you should consider the overall character of the orbital (sigma, pi etc) and the symmetry label of the orbital, relative contributions from AOs (and why they occur), the symmetry/point group of the molecule, and the constituent AOs (i.e. 2px/y/z, 1s, etc.). You've done different bits for each orbital comparison, but not all of them for every orbital.
Qualitative discussion of Aromaticity
Aromaticity is used to describe planar molecules with a delocalised electron pi system that exhibit more stability than other cyclic molecules that do not exhibit resonance. Bond lengths can give hints as to whether a molecule has a delocalised electron system as the bond length is in between the length of a single and double bond. Aromatic compounds play important roles in natural products and biochemistry of living organisms especially.
Hückel's rule can be used to determine whether a molecule classifies as aromatic. Hückel's rule states that a molecule must have a continuous ring a of p orbitals which can bond together to form a fully conjugated system of p orbitals, the molecule must be cyclic and planar and have 4n + 2 electrons (where n=integer value).
Molecules can have conjugation from overlapping pz orbitals, however this does not necessarily mean the molecule exhibits aromatic properties. Therefore overlapping pz orbitals alone is not a good description or criteria for aromaticity. Aromatic structures must all exhibit properties of resonance, however this does not imply that all molecules with resonance are aromatic structures. The real MO's portrayed above of benzene and borazine show sigma and pi bonding orbitals and thorough orbital combinations that surpass just pz orbitals overlapping.
Ng611 (talk) 14:13, 6 June 2018 (BST) A good basic description of aromaticity. Some thoughts on how the modern coneptual model of aromaticity differs from Huckel's model would improve the section further.
