Rep:Mod:splitinorganicprojectP2
Mini Project based on Ferrocene
For the project, I have chosen to study ferrocene. It consists of two cyclopentadienyl rings bound on opposite sides of the central Fe atom. The barrier for rotation of the Cp rings about the central Fe is very low so at room temperature, the rings are constantly rotating, between the staggered and the eclipsed form. I will start off with analysing the staggered and eclipsed form of ferrocene to study their relative stability. From this point, I aim to study the effect of changing the cyclopentadiene ligang by replacing the H with electron donating and electron withdrawing groups and analyse the natural bond order of the molecules.
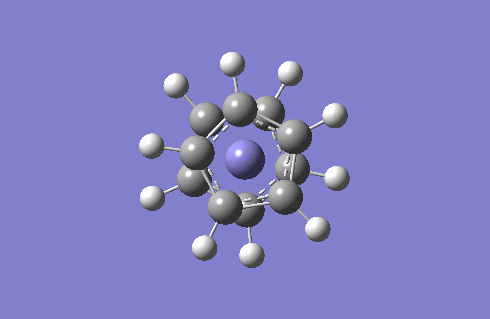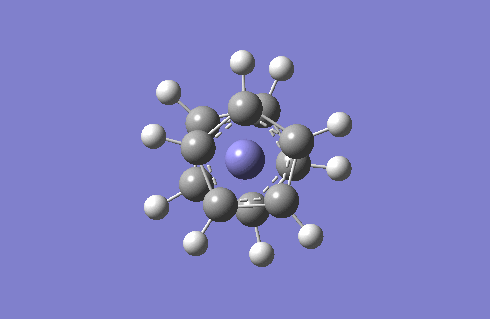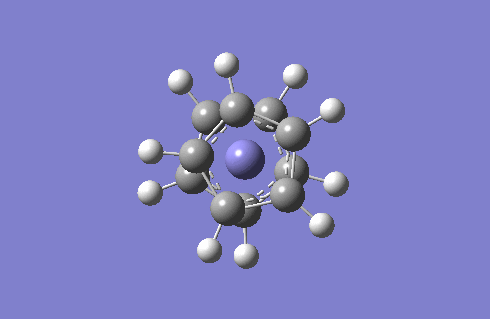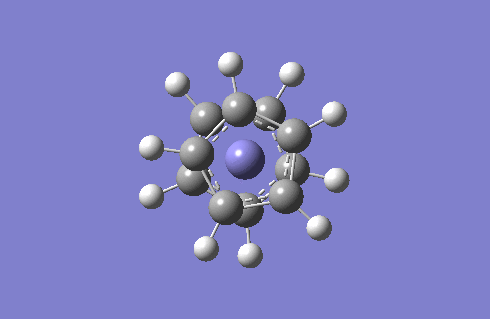
Staggered and Eclipsed Ferrocene
 |
 |
I will optimise the two structures of ferrocene and then carry out frequency analysis. Since there is a Fe atom to consider, I will use the high level basis set LANL2DZ and pseudo-potentials because the relativistic effects of Fe cannot be recovered by using the Schrodinger equation.
Frequency analysis is carried out to see if optimisation is complete and also provides us with the IR to compare. The IR spectra of the staggered and eclipsed ferrocene are shown below, eclipsed on the left and staggered on the right. The spectra look identical which proves that at room temperature, the Cp rings are constantly rotating so ferrocene adopts both the staggered and eclipsed form.

|
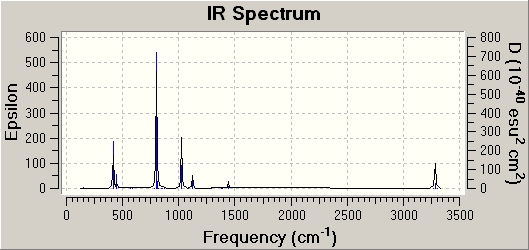
|
One very interesting fact to note is that there one negative frequency at -38cm-1 calculated for the staggered form. The frequency analysis is essentially the second derivative of the potential energy surface if one frequency is negative we have a transition state. When this vibration is animated, it can be seen that the ring above and below the Fe are rotating, such that they are trying to adopt the eclipsed conformation. Therefore the equilibrium conformation of ferrocene is eclipsed rather than staggered.
Staggered and eclipsed. Staggered is a Transition State, shown by freq analysis. One negative frequency.
Electron Withdrawing groups
Two groups, Cl and COR will be studied. They are both electron withdrawing groups but they do so via different methods. This is to be analysed by NBO analysis to see how this affects the charge distribution in the molecules
 |
 |
Electron Donating groups
One of the most common ligands to replace Cp in sandwich molecules if Cp*, in which the H on the cyclopentadienyl rings are substituted with a methyl group. These are electron donating groups which Cp* more electron rich than Cp. This will be studied by NBO analysis.
Another electron donating group which will be studied is OH. This donate
| No | Form of the vibration | Frequency | Intensity | symmetry of D2h point group |
|---|---|---|---|---|
| 1 | 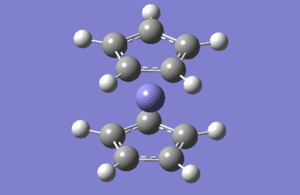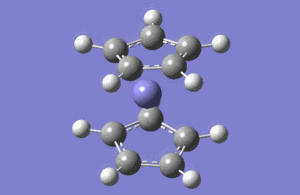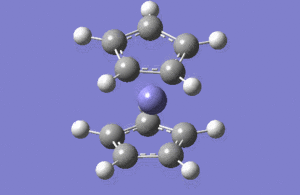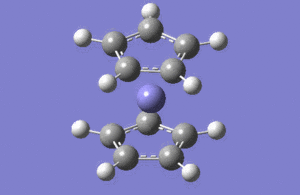 |
1146.03 | 92.87 | A2" |
| 2 | 1204.86 | 12.31 | ||
| 3 | 1204.86 | 12.31 | ||
| 4 | 2591.65 | 0.00 | Totally symmetric A1' | |
| 5 | 2730.07 | 103.74 |
References and citations