Rep:Mod:sp mod2
3rd Year Computational Lab: Module 2 - Bonding
Day 1
BCl3
The BCl3 optimisation described in the introduction was recreated for this report. The optimised molecule and the calculation summary, which contains all the requested information, are displayed below.
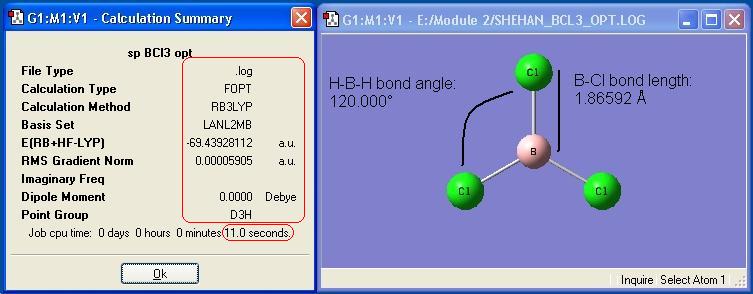
Small Molecule
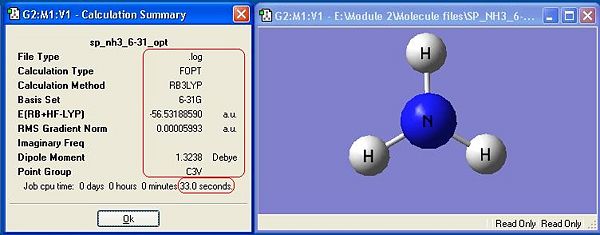
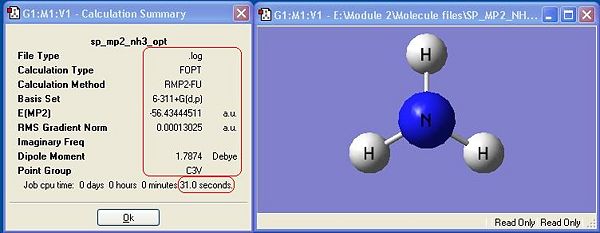
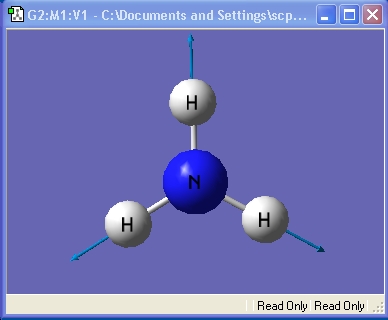
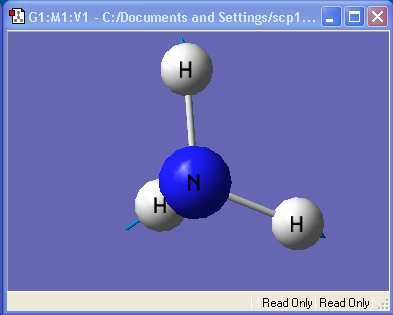
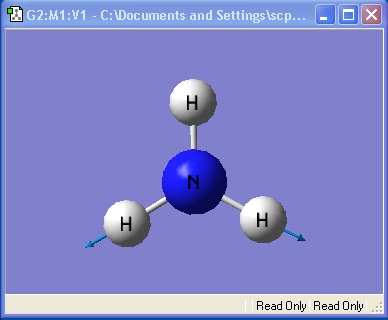
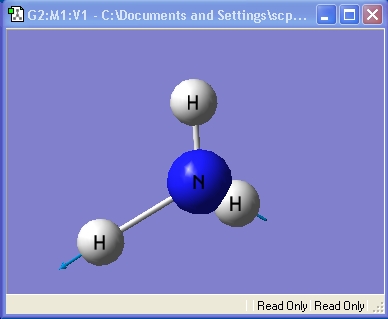
Ammonia, NH3,was chosen as the small molecule to optimise. The optimised molecule and the calculation summary, which contains all the requested information, are displayed below.
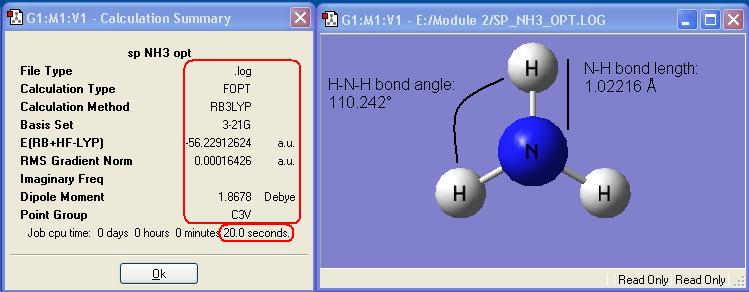
The xyz Cartesian co-ordinates were available in the .log file:

Published here:DOI:10042/to-1106
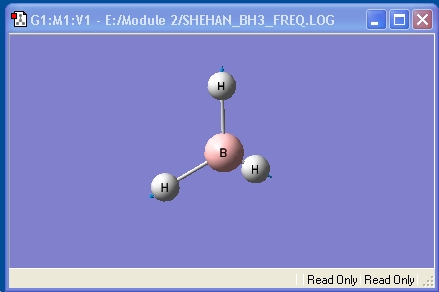
BH3
The BH3 optimisation described in the introduction was recreated for this report. The optimised molecule and the calculation summary, which contains all the requested information, are displayed below.
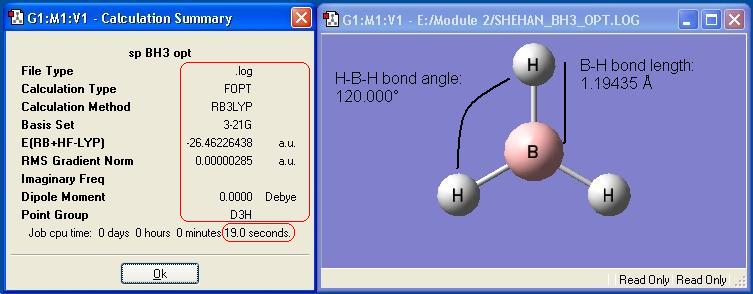
Vibrational Analysis of BH3
The vibrational analysis was carried out as instructed, and the information acquired is displayed in the table below:
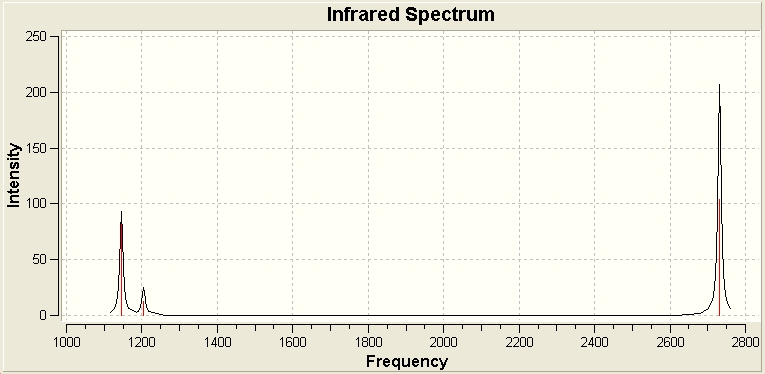
From looking at the table there are 6 defined vibrations. However, of these 6, there are two pairs of vibrations which have identical frequencies. This reduces the expected number of peaks from 6 to 4. Also from the table, we can see that vibration 4 has an intensity of 0. This vibration therefore does not show up on the spectra. The reason that this particular vibration is not observed is that there is no change in dipole. This leaves the 3 vibrations observed in the spectra.
Molecular Orbital Analysis of BH3
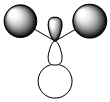
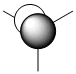
The quantitative Molecular Orbitals are displayed with their respective qualitative Molecular Orbitals in the table below
| Molecular Orbital | 1 | 2 | 3 | 4 | 5 |
| Qualitative Orbital | 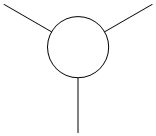 |
 |
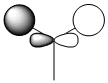 |
 |
 |
| Quantitative Orbital |  |
 |
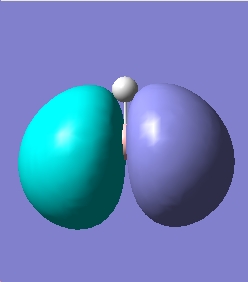 |
 |
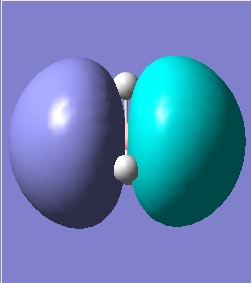 |
| Orbital Energy | -6.730 | -0.517 | -0.356 | -0.356 | -0.074 |
Talk about energy
Using the available literature [1], the following MO diagram was produced in ChemDraw:
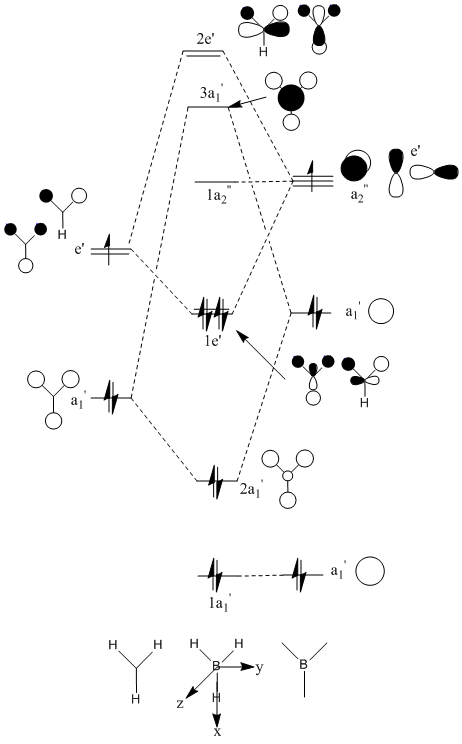
From looking at both the qualitative and quantitative MOs it is clear that they correlate very well, both in shape and electron density. Although when comparing the two sets of Mos, we must bear in mind that the qualitative MOs consist of linearly combined Atomic Orbitals. Where we see multiple AOs in a qualitative MO, they construcitvely (in pahse) overlap to form single lobes, we expect (and observe) a single 'phase' lobe/orbital in the quantitative MO. Where we see destructively (out of phase) overlap, we expect (and observe) nodes in the quantitative MO.
The fact that the qualitatitive and quantitative MOs correlate so well (at least for low energy MOs in a simple inorgnic molecule) indicates that qualitative MO theory is a good model, as it can quickly and accurately predict MOs without the need for a computer/calculations. It would be worth investigating whether MO theory holds up well ifapplied to more complex molecules, such metal complexes, aromatic systems, multicentre multielectron bonds (e.g. B2H6). cf VESPR to MO.
Day 2
Isomers of Mo(CO)4L2
The cis and trans isomer were initially optimised using the B3LYP method with a LANL2MB basis set, with the convergence criteria 'opt=loose'. After this, it was further optimised using the LANL2DZ basis set, with the electronic convergence 'int=ultrafine scf=conver=9'. Both optimisation were done using SCAN. Once the fully optimised geometries were returned from SCAN, vibrational frequency calculations were run on each isomer. The obtained results, as well as the fully optimised 3D molecule for each isomer are displayed below. To calculate the energy of each isomer in kJ mol-1, it was converted from Hartrees to Joules, then scaled up to a mole:
EJ=(EH x 4.359 744 17 x 10-18 x 6.0221415 × 1023)/1000
where EJ is energy in Joules and EH is energy in Hartree
| Isomer | Fully optimised cis-Mo(CO)4PMe3[2] | Fully optimised trans-Mo(CO)4PMe3[3] | ||||||
| 3d Molecule |
|
| ||||||
| IR Spectra | 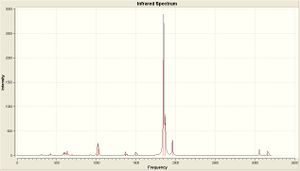 |
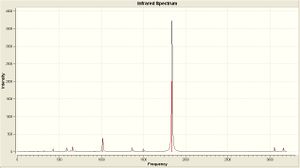 | ||||||
| Calculated Frequency (cm-1) | 1848.43/1850.53/1869.89/1960.3 | 1839.5/1839.29 | ||||||
| Literature[6] Frequency (cm-1) | 1882/1903/1917/2022 | 1901 | ||||||
| Energy (kJ mol-1) | -2030457.024 | -2030449.336 |
From the information above we can conclude that the trans isomer is more stable. This would be expected[6] due to the reduction in steric interaction of the PR3 groups when in the trans isomer. The energy difference is calculated as 7.688 kJ mol-1.
As is clear from the IR there is only one dominant peak in each spectra (at about 1900 cm-1) which corresponds to the C=O stretch. It is also clear that there are numerous peaks for this range in the cis isomer spectra, where the trans spectra has a well defined peak. This peaks have been defined above in the table, as well as the corresponding literature value (or as close as I have been able to tell). The vibrational frequency for the trans isomer compares favourably, and is within 3%. The cis isomer compares equally favourably, with all the calculated frequencies within 3%.
| Isomer | Mo-P Calculated Bond Length (Å) | Mo-P Literature Bond Length (Å) | Mo-C Calculated Bond Length (Å) | Mo-C Literature Bond Length (Å) | P-Mo-P Calculated Bond Angle | P-Mo-P Literature Bond Angle |
| cis isomer[7] | 2.652/2.648 | 2.522 | 2.032/1.981/1.983/2.031 | 2.042/1.966/1.975/2.022 | 95.31° | 97.54° |
| trans isomer[8] | 2.572 | 2.500 | 2.028/2.029 | 2.005/2.016 | 179.83° | 180° |
In both the literature reports the complex was of the form Mo(CO)4(PR3)2. For the cis isomer R=Me (the exact same molecule, so we can expect better correlation between calculated and experimental values) but for the trans isomer R=Ph, so we may expect slightly more disparity between results. Unexpectdely it is the trans isomer that correlates better with its experimental values, the P-Mo-P bond angle is very close to the experimentally measured angle. All the bond lengths are within 3% of the experimentally measured ones.
In the cis isomer the differences in bond lengths and angle are greater; 5% and 2.4% respectively. In both isomers the best match is between the Mo-C bonds (difference ~1%) and the Mo-P bonds show the greater difference (3-5%). Since the d orbitals are not used in he calculations the bonds with the lowest d character (Mo-C)are the best predicted, whereas the Mo-P bond which has a lot of d character is (relatively) poorly predicted.
However, both isomers are within a reasonble degree of accuracy considering that a medium level basis set/method was used, and that the d orbitals were not included in the calculations. Had the advance method/basis set been used (which included the d orbitals), I would expect the bond lengths/angle to be even closer to the literature value.
It may be possible to alter the relative ordering of the cis/trans isomers by adjusting the ligands steric and electronic properties. By adding large steric groups, or EWG/EDG it would be possible to alter the steric and electronic factors repectively. There is the possibility that such interactions can activate or improve a metal's ability to act as a catalyst. Catalysts are often improved by making slight changes to secondary groups on the ligands themselves, while leaving the metal center/bonding ligand intact. Additionally, where regiospecific catalysts are required, groups on ligands can be selected as to favour a specific isomer.
Day 3
Quantum Nature of Ammonia
Symmetry
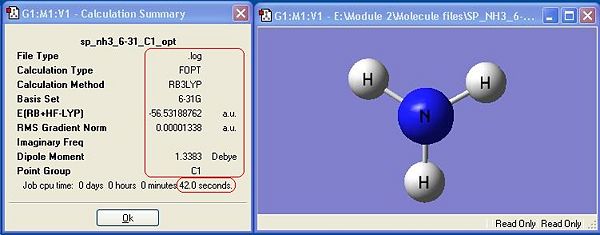
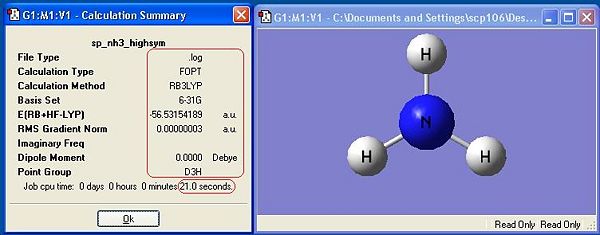
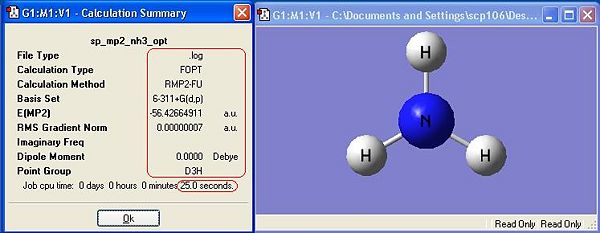
The symmetry has made a difference to the final structure obtained. This is shown by the differences in energy between the first 3 molecules. There is a distinct, albeit very small difference in energy, with C1 being the lowest, thus most stable symmetry.
The general trend observed is that the higher the symmetry, the quick a molecule is optimised. This is because symmetry must be retained during the calculation, so as the programme optimises the geometry, it has fewer possible optimisation steps because symmetry must be retained. There are statistically fewer different co-ordinations the molecule can be arranged in while remaining symmetrical.
During the calculation symmtery must be retained, and cannot be broken unless specifically specified (the tickable 'ignore symmetry' box in Gaussview). This means that high symmetry structure optimise more quickly.
The lowest energy geometry is C1, whereas the highest symmetry D3h is the highest in energy. The difference in energy between these two geometries is 0.9007 kJ mol-1. The difference in energy between these two geometries is very low, and at room temperature there is sufficient energy to overcome it. This means that Ammonia is constantly switching between the two geometries in an umbrella type motion. From equipartition theory (T=298K):
E= 3/2 kT E(per mol) = 3/2 RT E= 3.716 kJ mol-1
The calculations for the better basis set/method took on average longer. However, since the laptops can be 'temperamental' the above data isn't the most conclusive evidence of this we are likely to see. However, were slightly larger molecules considered, the better basis set/method calculations would take longer.
The energy difference calculated (20.4684 kJ mol-1) compares quite well with the experimentally determined energy barrier (24.3 kJ mol-1). There is a 16% difference between the energies. The energy difference for this basis set is much greater than the energy differenec for the previously used basis set.
Vibration
| Point Group | C3v | D3h |
| IR Spectra | 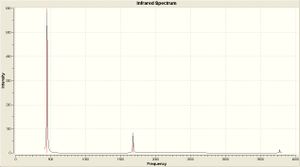 |
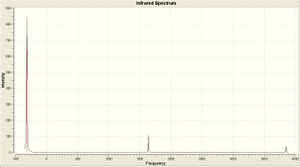 |
| Computational Frequency(cm -1) | 452.302 | 1680.47 | 1680.47 | 3575.43 | 3775.76 | 3775.76 |
| Experimental[9] Frequency(cm -1) | 932 | 1626 | 1626 | 3337 | 3444 | 3444 |
The first frequency has a very large difference of ~50%. However, the remaining 5 are within 9% of the literature values.
Mini Project
Fuels of the Future
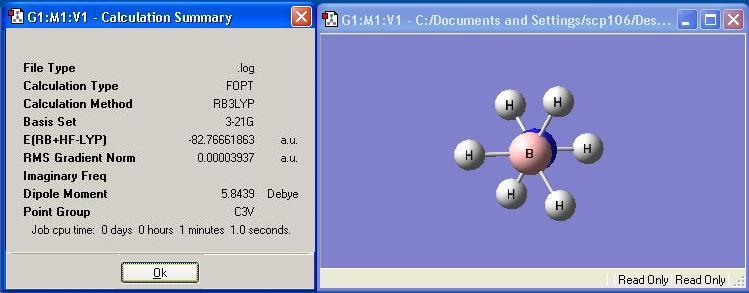
Staggered BH3NH3 was optimised and the calculation summary is displayed below:
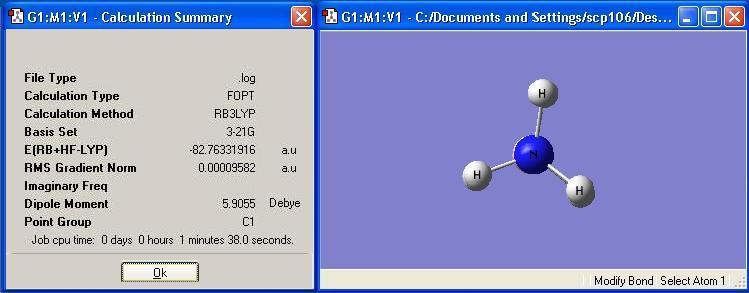
Eclipsed BH3NH3 was optimised and the calculation summary is displayed below:
The staggered conformation is the lowest in energy, thus most stable form. The energy difference between the two conformations is 8.663 kJ mol-1.
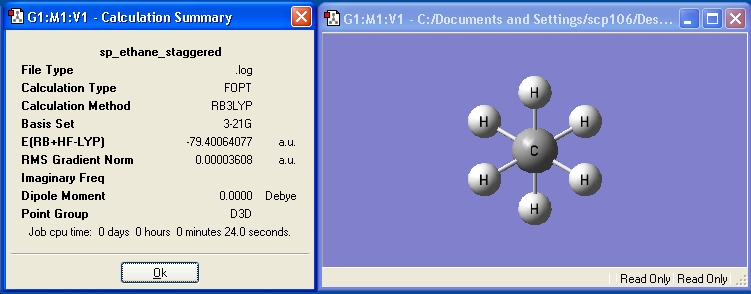
Staggered ethane was optimised and the calculation summary is displayed below:
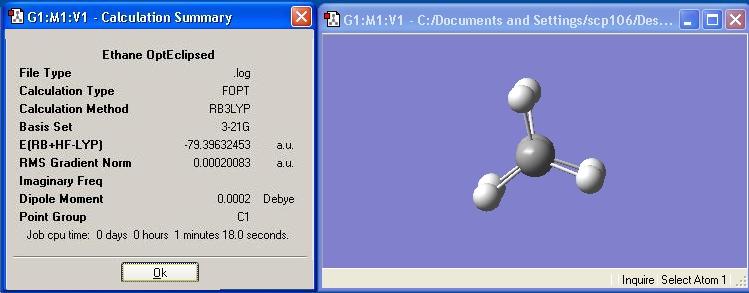
Eclipsed ethane was optimised and the calculation summary is displayed below:
Again the staggered conformation is the lowest in energy, thus most stable form. The energy difference in this case is 11.327 kJ mol-1. This energy difference is some 30% larger than the respective difference between conformers of ammonia borane.
In ethane we expect the electron density of the C-C bond to be evenly spread, and all the C-H bonds to be equal. However, in ammonia borane the electron density of the N-B bond would be more around the N atom, as Nitrogen is more electronegative. This would also cause the N-H bonds to be shorter than the B-H bonds (0.96Å vs 1.15Å [10]).
Ammonia borane is lower in energy than both starting product. Additionally, the formation of Hydrogen gas provides an entropic driving force for the reaction.
The large difference in melting points between ethane and ammonia borane is due to the highly polar nature of the molecule. The hydrogen atoms bonded to nitrogen are acidic in character, whereas the hydrogens bonded to boron are basic in nature. Studying the solid structure has indicated "close association of the NH and BH centres"[11].
References
- ↑ Lecture Notes from http://www.ch.ic.ac.uk/hunt/teaching/teaching_MOs_year2/
- ↑ unable to publish
- ↑ Published hereDOI:10042/to-1107
- ↑ Published hereDOI:10042/to-1108
- ↑ unable to publish
- ↑ 6.0 6.1 D. J. Darensbourg, Inorg. Chem., 1979, 18, 14-17DOI:10.1021/ic50191a003
- ↑ trans isomer: G. Hogarth and T. Norman, Inorganica Chimica Acta, 1997, 254, 167-171 DOI:10.1016/S0020-1693(96)05133-X
- ↑ cis isomer: F. A. Cotton, D. J. Darensbourg, S. Klein and B. W. S. Kolthammer, Inorg. Chem., 1982, 21, 2661-2666 DOI:10.1021/ic00137a026
- ↑ D. A. Dixon and M. Gutowski, J. Phys. Chem. A, 2005, 109, 5129-5135DOI:S1089-5639(04)04562-1 10.1021/jp0445627 S1089-5639(04)04562-1
- ↑ Data from http://en.wikipedia.org/wiki/Ammonia_borane
- ↑ Study of the N-H···H-B Dihydrogen Bond Including the Crystal Structure of BH3NH3 by Neutron Diffraction DOI:S0002-7863(98)02533-5 10.1021/ja9825332 S0002-7863(98)02533-5