Rep:Mod:sp31
Introduction
Using Molecular mechanics and semi-empirical Molecular Orbital methods, the reactivity and structure of molecules can be predicted without having to actually do the chemistry. There are many limits to these models but for simple molecules they give fairly accurate results, which can then be used to rationalise various aspects of their chemistry.
Molecular Mechanics treats all the atoms as balls and the bonds as springs. It calculates the energy by adding up the energy terms of all the different interactions. The different interactions it cconsiders are[1]:
- Bond stretching: this takes into consideration how far from its ideal length a bond is. The further away it is the higher the energy.
- Bending: this is a measure of the angle between to atoms that are geminal to each other, Changes from the optimal energy affect the energy.
- Torsion: this relates to the barrier of rotation for a bond.
- Non-bonded interaction:atoms that aren't bonded to each other also interact, through Van der Waals interactions, steric repulsion and electrostatics.
- H-bonding: this is optional but hydrogen bonding plays a large role in dertmining the geometry of a molecule.
In order to see Jmols of the molecules, click on the links below the images or in the tables.
Hydrogenation of Cyclopentadiene Dimer

Cyclopentadiene readily dimerises to give the endo product 2 as oppose to the exo product 1. Hydrogenation of the dimer initially gives either product 3 or 4 but prolonged hydrogenation is needed to give the tetrahydro product.[2]
The geometry of the two Cyclopentadiene dimers were definined using ChemBio 3D and otimised using the MM2 force field option. The energy of the two dimers was calculated to be 133.37KJmol-1 and 142.25KJmol-1respectively. This says that the thermodynamic product is the exo product 1. Therefore as the product that is formed is the kinetic product, the reaction must be kinetically controlled and go via the lowest energy tranisition state.
| Property | 1 | 2 |
|---|---|---|
| stretch /KJcal-1 | 1.28 | 1.25 |
| bend /KJcal-1 | 20.57 | 20.84 |
| Torsion/KJcal-1 | 7.6 | 9.51 |
| 1,4 VDW /KJcal-1 | 4.23 | 4.31 |
When looking at the seperate components it can be seen that the biggest difference in the energies of the two molecules is in the torsional strain component. This means that 2 deviates more from normality than 1,ie. 2 has more eclipsed conformations than 1.

The dimerisation of Cyclopentadiene occurs via a Diels-Alder cycloaddition, using a frontier orbital method it can be seen why the endo product forms. Figure 2 shows the homo and lumo interacting with the dashed lines showing where the new bond will form, with the alignment for the endo product. As the bond forms the other orbitals are brought together which stabilises the transition state, lowering its energy.


The two products of hydrogenation 3 and 4 (figure 1)were also defined and optimised. This gave energies of 154.22KJmol-1 and 130.34KJmol-1 respectively, this shows that 4 is the thermodynamic product. As can be seen in the table below, product 4 has lower values for all the contributions of stretching, bending, torsion and H-Bonding energy contributions. This is therfore the double bond in the norborene part of the ring is hydrogenated first under normal conditions, to give product 4. It then takes more forcing conditons to hydrogenate the double bond in the cyclopentane ring.
| Property | 3 | 4 |
|---|---|---|
| stretch /KJmol-1 | 5.18 | 4.59 |
| bend /KJmol-1 | 78.58 | 60.766 |
| Torsion/KJmol-1 | 53.22 | 52.29 |
| 1,4 VDW /KJmol-1 | 25.32 | 18.88 |
| Total Energy /KJmol-1 | 154.22 | 130.34 |


Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
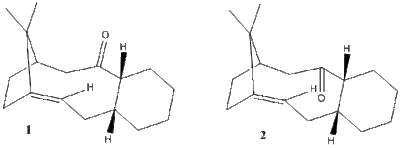
Taxol is an important drug in the treatment of ovarian cancer. A key intermediate 1 or 2 in Figure 3 in its synthesis can be synthesised with the carbonyl pointing up or down. However when left to equilibrate, the more stable isomer dominates. The two molecules are atropisomers, conformers that can be isolated, due to the energy barrier of the rotation of the bond, being too high for the molecule to overcome at room temperature.
Using chemdraw3D and the MM2 force field the energies of the 2 molecules was calculated. this gave energies of to be: 221.7 KJmol-1 for 1 and 178.6 KJmol-1 for 2. This gives a difference in energy of 43.1 KJmol-1. When the MMFF94 force field was used the two energies were 267.5 and 326.18 KJmol-1 respectively. This gives an energy difference of 58.7KJmol-1. Therefore it can be presumed that 1 isomerises to 2 when left standing as two is lower in energy. When the carbonyl group is up, it is on the same side as the bridge. This brings one isopropyl group very close to the oxygen and also causes the two ends of the molecule to be pointing towards each other, whereas when the carbonyl group is down the molecule has a slightly more open structure.
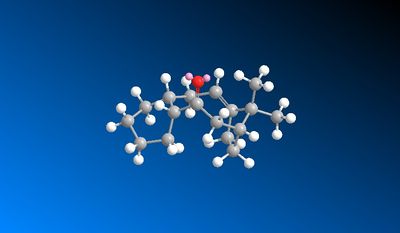
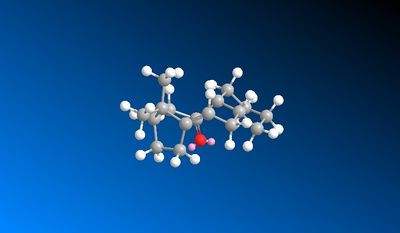
In the subsequent functionalisation of the alkene, the reaction only goes slowly. This is because the alkene is a hyperstable alkene. A hyperstable alkene is a bridgehead trans alkene in a midsized ring, usually of 8 or more carbons. They have unusually low olefin strain values. They are unreactive due to their stability, which is why the functionalisation reaction is slow..[3]
Semi-empirical Molecular Orbital Theory: Regioselective addition of dichlorocarbene
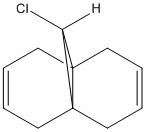
The compound in figure 4 reacts regiospecifically with electrophiles at only one of the double bonds. The reason for this can't be explained using a mechanical method and instead requires an approach that considers the orbitals. The structure was optimized using MM2 and then the MOPAC PM6 MO method was run to give an approximation of the molecular orbitals. From this the HOMO, LUMO and surrounding orbitals can be analysed.
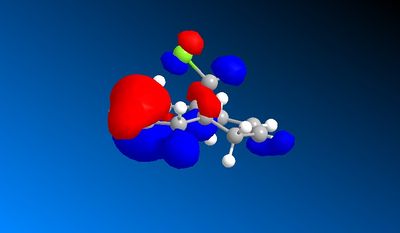
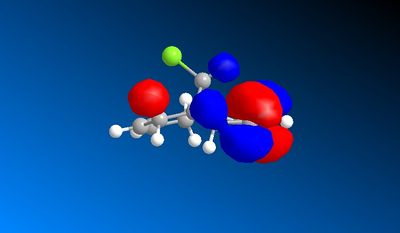

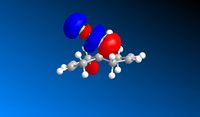
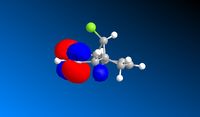
An Electrophile such as dichlorocarbene will donate electrons into the HOMO orbital. In this compound the HOMO is mostly centered around the double bond endo to the chlorine atom. Therefore this bond is the one that would react with the dichlorocarbene.
The orbitals were also calculated using the RM1 force field, this gave similar looking orbitals for the HOMO and LUMO but the other orbitals were different. However when more orbitals were looked at it became apparent the same orbitals were present but in a different order, for instance the PM6 HOMO-1 matched the RM1 HOMO-3. RM1 is a less powerful forcefield, so the PM6 optimised structure was used for interpretation and futher calculations.
Using Gaussian the vibrational motion of the molecule can be analysed[4][5]. This can be then be compared to the monohydrogenated version where the exo double bond has been replaced by a single bond. In the monohydrogenated version all the vibrations are at lower wavelengths, than in the dialkene. A normal vibration for a double c-c bond is usually at about 1680-1620 cm-1. This is lower than either of the stretches calculated. Therefore the C-Cl bond must be increasing the strength of the double bond, causing it to vibrate at higher frequencies.
Monosaccaride chemsistry: Glycosidation
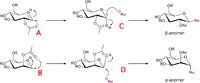
Glycosidation is the reaction of a sugar with a nucleophile. Depending on the orientation of the adjacent acetoxy group, different anomers form, with very good control. This is due to Neighbouring Group Participation which is depicted in figure 5. To form the alpha anomer the acetyl oxygen must attack from the top so that the nucleophile has to attack from the bottom face. To form the beta anomer the oxygen has to attack from the bottom so that the nucleophile attacks from the top face.
When using Chemdraw3D the R group was assigned as a methyl group. This is because having a small group reduces the computational demand. However using a hydrogen may not fully represent the chemistry involved as different orbitals are involved in the bonding. The geometry was optimised using both the MM2 and MOPAC PM6 force fields and the results are reported below.
| A | A' | B | B' | C | C' | D | D' | ||
|---|---|---|---|---|---|---|---|---|---|
| MM2 KJmol-1 | stretch | 11.9 | 10.6 | 9.62 | 10.3 | 8.32 | 11.35 | 8.45 | 11.2 |
| bend | 57.8 | 38.8 | 40.3 | 42.2 | 54.2 | 70.34 | 66.9 | 84.9 | |
| stretch-bend | 5.31 | 3.70 | 3.55 | 3.62 | 3.10 | 3.23 | 3.02 | 3.29 | |
| torsion | 21.6 | 15.98 | 7.61 | 0.67 | 33.2 | 32.37 | 32.8 | 24.5 | |
| non 1-4 VDW | -9.75 | -8.23 | -14.4 | -7.50 | -16.2 | -11.2 | -9.72 | -10.9 | |
| 1,4 VDW | 79.4 | 84.1 | 79.9 | 78.5 | 73.81 | 27.6 | 81.3 | 76.3 | |
| charge/dipole | 28.3 | -46.5 | 5.61 | -31.4 | 5.24 | -2.0 | -25.8 | -15.6 | |
| dipole/dipole | 26.1 | 22.2 | 16.4 | 20.8 | 1.62 | -3.13 | 0.63 | 2.03 | |
| total energy | 220.7 | 120.7 | 148.6 | 117.3 | 163.3 | 182.3 | 151.5 | 175.7 | |
| MOPAC PM6 KJmol-1 | -289.4 | -358.7 | -288.7 | -324.3 | -350.2 | -279.2 | -327.2 | -274.3 |
The PM6 and MM2 Methods give very different values. The differences in values between the different conformers for the two different methods is detailed in the table below. The PM6 values are negative but show the same trends as the MM2 although the energy gaps vary significantly. The PM6 values are probably more accurate as they begin to take into account the orbitals involved as oppose to just treating the molecule as balls and sticks. Also in a similar study by D Whitfield and T Nukada[6], using DFT methods, the energies they obtain are also negative.
| A/A' | B/B' | C/C' | D/D' | |
|---|---|---|---|---|
| MM2 | 100.0 | 31.3 | -19.0 | -24.2 |
| PM6 | 69.3 | 35.6 | -71.0 | -52.9 |
For the starting sugars, the A/A' and B/B' pairs, the confomer, A' or B', where the acetyl oxygen is on the same side of the ring bond has a higher energy. However in the intermediate, when the 5 membered ring is formed, the confomer where both C-O bonds are on the same face, C and D, have a much lower energy than when the 5 membered ring is wrapped around the hexose ring. Because this conformation is more stable, these intermediates are more likely to remain in the reaction pot and so can be attacked by the nucleophile. This then determines the stereochemistry. So although A' is the preferred conformation, the intermediate C is more likely to form and therefore the product will be the beta anomer.
MiniProject: α arylated, β substituted cyclopropylphosphonates
Cis Isomer |
Cis Isomer
Trans Isomer |
Trans Isomer
α arylated, β substituted cyclopropylphosphonates can be made electrosynthetically from diethyl alpha, alpha-dichlorobenzylphosphonate and methyl acrylate. The reaction is an electroreduction performed in DMF. The diastereoselectivity depends on the michael acceptor used, and methyl acrylate gives a mixture of the Trans and the Cis isomers, but the Cis isomer is the major isomer. The two different isomers were determined by measuring 3JCP coupling constants. The coupling constant for cis configurations are higher than those for trans configurations.[7] The two structures were optimised using gaussian[8] and then 13C NMR spectrum was predicted and compared with the values in the literature[9][10]. The values for carbon 4 were corrected using 0.96x(value)+ 12.2, as gaussian tends to be out for the carbon of a carbonyl.
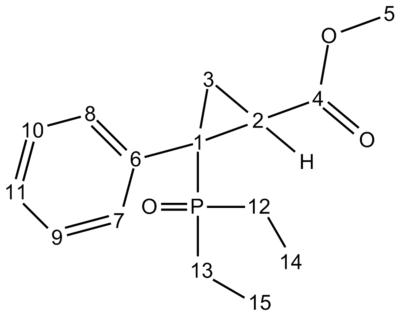
| Carbon | Gaussian Cis δ/ppm | Lit. Cis δ/ppm | Gaussian Trans δ/ppm | Lit. Trans δ/ppm |
|---|---|---|---|---|
| 1 | 33.3 | 30.4 | 34.5 | 30.3 |
| 2 | 32.7 | 28.9 | 28.0 | 24.9 |
| 3 | 16.5 | 16.7 | 18.5 | 16.4 |
| 4 | 169.9 | 170.0 | 168.7 | 170.0 |
| 5 | 51.4 | 52.6 | 50.4 | 52.3 |
| 6 | 133.4 | 139.1 | 129.0 | 134.2 |
| 7 | 132.5 | 130.9 | 128.5 | 131.2 |
| 8 | 125.2 | 130.9 | 128.5 | 131.2 |
| 9 | 124.6 | 128.7 | 124.7 | 128.5 |
| 10 | 124.6 | 128.7 | 124.7 | 128.5 |
| 11 | 124.5 | 128.0 | 124.5 | 128.1 |
| 12 | 57.3 | 62.7 | 58.9 | 63.2 |
| 13 | 61.8 | 63.1 | 61.5 | 63.3 |
| 14 | 16.0 | 16.7 | 16.5 | 16.7 |
| 15 | 17.0 | 16.7 | 16.7 | 16.7 |
In order to see whether the experimental values for the Cis compound was a better fit for the Literature values for the cis or trans compound and vice versa, the experimental value was taken away from the Literature value to see which one has the smallest deviation.
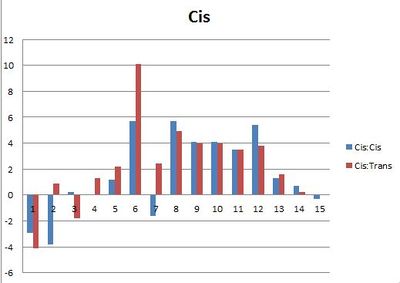
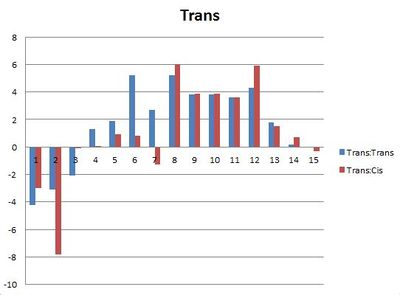
Ideally one of set of bars would be considerably smaller than the other, as then it could definitvely be said that this could be used to distinguish between the isomers. However this isn't the case, most of the bars are very similar in height or the smaller difference is between the wrong sets of data eg cis and trasn as oppose to cis and cis. The most dramatic deviation is at carbon 6. This is the ipso carbon of the aromatic ring, for both the experimental and literature values the cis compound has a higher chemical shift. In the trans configuration, the aromatic ring is aligned with the oxgen of the ester and there will be some orbital interaction, which lowers the chemical shift. This isn't present in the cis configuration so there is a large difference between the values. Because the experimental spectra don't definitively match one isomer or the other, they couldn't be used to determine which isomer an unknown compound is. This may be due to the program, struggling with the molecule. Three membered rings are very strained and therefore can have unusual molecular orbitals. The phosphorus atom also complicates matters. The Phosphorus NMR can also be calculated. Both spectra have a single peak but the shift values are 358.0ppm for the trans isomer and 352.3ppm for the cis isomer, the literature values are 25.5 and 24.2 ppm respectively, so something is clearly wrong. The original literature uses 3JCP coupling constants in order to distinguish between the two isomers, as the cis isomer has a larger coupling constant.[11] However with the Phosphourus NMR being unreliable, it is not worth the computational demand continuing to use this program. It may be that other programs give better results for this system, which could be tried in futher experimentation.
References
- ↑ http://classweb.gmu.edu/sslayden/Chem350/manual/docs/MM.pdf
- ↑ Kinetics of Dicyclopentadiene Hydrogenation,D skala, Petroleum and coal, 2003, 45, 103-108.
- ↑ Hyperstable Olefins: Further calculational explorations and predictions, A. McEwen, P. Schleyer J. Am. Chem. Soc., 1986, '108, 3951-3960.
- ↑ Calculation data for Monoalkene DOI:10042/to-10328
- ↑ The calculation for the dialkene was run on my laptop so the data can't be published in d-space. Input: https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:Q3pm6dialkene.gjf Output:https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:3pm6(1).out
- ↑ DFT studies in the role of C-2-O-2 bond rotation in neigbouring group glycosylation reactions,D Whitfield, T.Nukada Carbohydrate reserch, 2007, 10, 1291-1304.
- ↑ Electrosynthesis of α-Arylated β-Substituted Cyclopropylphosphonates. Synthesis of a Phosphonic Analogue of Minalcipran,C. Duquenne, S. Goumain, P. Jubault, C. Feasson,J. Quirion. Org. Lett. 2000, 4, 453-455
- ↑ Calculation Data. Cis:DOI:10042/to-10330 Trans:DOI:10042/to-10329
- ↑ Literature Supporting Data: http://pubs.acs.org/doi/suppl/10.1021/ol991301k/suppl_file/ol991301k-5_s1.pdf
- ↑ Calculation Data. Trans:DOI:10042/to-10352 Cis:DOI:10042/to-10351
- ↑ J. Thiem, B. Meyer, Org. Mafn. Reson.. 1978, 11, 50