Rep:Mod:soocho12
Constructing BH3 molecule
B3LYP/3-21G level
Optimisation log file here
Optimisation
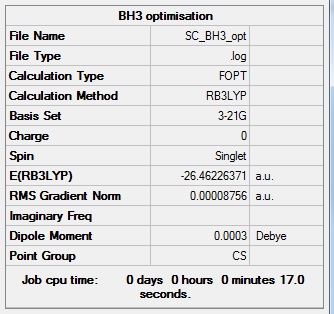
BH3:B3LYP/6-31G(d,p)level
Optimisation log file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|

|
Item Value Threshold Converged? Maximum Force 0.000070 0.000450 YES RMS Force 0.000039 0.000300 YES Maximum Displacement 0.000356 0.001800 YES RMS Displacement 0.000214 0.001200 YES |
|
GaBr3:B3LYP/LANL2DZ
Optimisation log file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|

|
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000003 0.001800 YES RMS Displacement 0.000002 0.001200 YES |
|
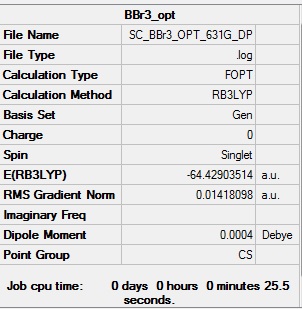
BBr3:B3LYP/LANL2DZ
Optimisation log file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|
|
Item Value Threshold Converged? Maximum Force 0.000011 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000035 0.001800 YES RMS Displacement 0.000029 0.001200 YES |
|
Geometry Comparison
| BH3 | GaBr3 | BBr3 | |
|---|---|---|---|
| r(E-X) Å | 1.20 | 2.39 | 2.02 |
| θ(X-E-X) degrees(º) | 29.90 | 30.00 | 119.99 |
BH3 and GaBr3 have 30º bond angles which is not quite right for D3H molecule. This shows that the molecules have not optimised properly. BBr3 has bond angle of 120º expected from D3H molecule. The bond lengths of 3 molecules are different to each other as they have different central atoms and the ligands.
BH3 and BBr3 have different bond lengths due to different size and electronegativity of ligands. Bromine is highly electronegative atom which pulls the electron density towards itself. Bromine's outermost electron is occupied in 4p orbital which is much more diffuse and larger than 1s orbital of hydrogen, therefore it interacts more strongly with Boron atom. Also, as Br's 4p orbital is partially filled, it is available for back-donation into empty B 2p orbital which gives more interaction with Boron atom. This gives B-Br bond length smaller than expected value.
GaBr3 and BBr3 have different bond lengths due to different central atoms. Ga is much heavier atom than B with more electrons occupying in more diffuse 4p orbitals. Br also has valence electrons occupied in 4p orbitals and the interaction between 4p-4p of Ga-Br would be more stronger than that of 2p-4p B-Br due to orbital match, resulting in stronger bonding.
Chemical bond is an attraction between atoms allowing the formation of chemical molecule by transferring and sharing electrons as well as electrostatic forces.
Strong bonds are 'intramolecular' the chemical bonds within a molecule. The bond is made by transferring or sharing electrons between atoms and depends on the electrostatic force between protons in nucleus and electrons in the orbit. This includes ionic, covalent and metallic bonding.
Weak bonds are 'intermolecular' forces which is an attraction and repulsion between molecules that hold molecules, ions, and atoms together. This includes hydrogen bonding, dipole-dipole interactions or London dispersion.
When we first optimised the molecule, the bond is not shown because there is a pre-defined value and if the bond lengh exceeds this value (in inorganic view), it does not appear.
Frequency Analysis
BH3:B3LYP/6-31G(d,p)
Frequency file: here
| summary data | low modes |
|---|---|
|
Low frequencies --- -12.4035 -12.3969 -7.7522 -0.0008 0.0236 0.4043 Low frequencies --- 1162.9690 1213.1353 1213.1355 |
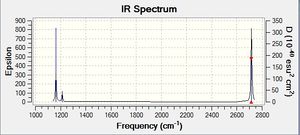
Vibrational spectrum for BH3
| wavenumber | Intensity | IR active? | type |
| 1163 | 93 | yes | bend |
| 1213 | 14 | very slight | bend |
| 1213 | 14 | very slight | bend |
| 2583 | 0 | no | stretch |
| 2716 | 126 | yes | stretch |
| 2716 | 126 | yes | stretch |
GaBr33:B3LYP/6-31G(d,p)
Frequency file: here
| summary data | low modes |
|---|---|

|
Low frequencies --- -1.4878 -0.0015 -0.0002 0.0096 0.6540 0.6540 Low frequencies --- 76.3920 76.3924 99.6767 |
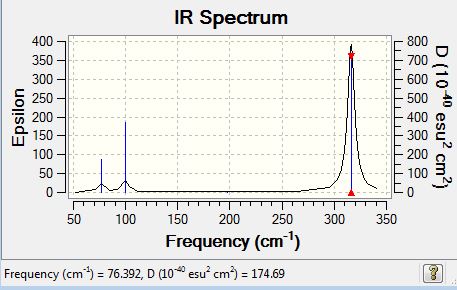
The frequencies for GaBr3 are all significantly lower than those of BH3 as both the central atoms and ligands are higher in mass than the atoms of BH3. As the mass of the molecule increases, the vibrational frequency decreases. The frequency is inversely proportional to the square root of reduced mass of atoms in the molecules.
Vibrational spectrum for GaBr3
| wavenumber | Intensity | IR active? | type |
| 76 | 3 | no | bend |
| 76 | 3 | no | bend |
| 100 | 9 | slight | bend |
| 197 | 0 | no | stretch |
| 316 | 57 | yes | stretch |
| 316 | 57 | yes | stretch |
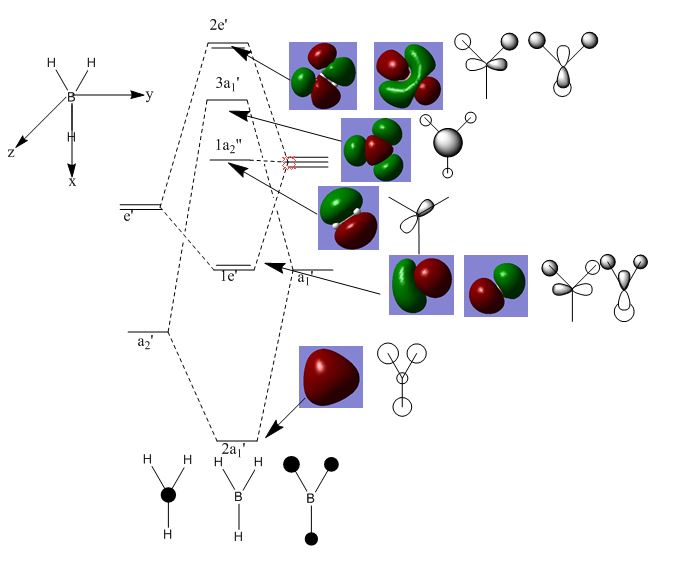
BH3 Molecular orbital diagram
NH3 Analysis
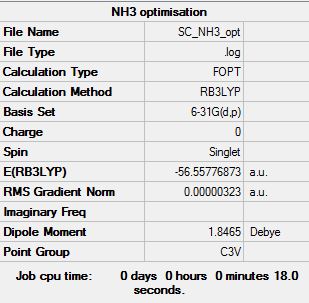
Optimisation
Optimisation log file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|
|
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES |
|
Frequency
Frequency file: here
| summary data | low modes |
|---|---|
|
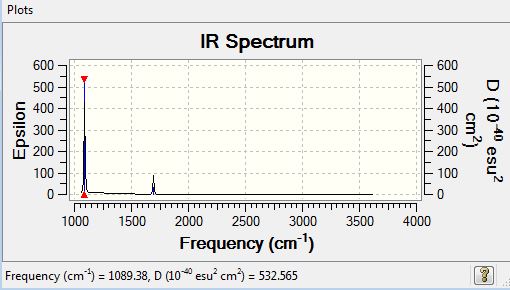
Low frequencies --- -0.0139 -0.0031 -0.0009 7.0783 8.0932 8.0937 Low frequencies --- 1089.3840 1693.9368 1693.9368 |
Vibrational spectrum for NH3
| wavenumber | Intensity | IR active? | type |
| 1089 | 145.4 | yes | bend |
| 1694 | 13.6 | slight | bend |
| 1694 | 13.6 | slight | bend |
| 3461 | 1.1 | no | stretch |
| 3590 | 0.3 | no | stretch |
| 3590 | 0.3 | no | stretch |
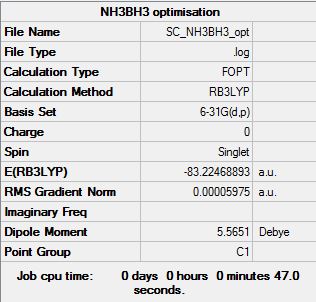
NH3BH3 Analysis
Optimisation
Optimisation log file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|
|
Item Value Threshold Converged? Maximum Force 0.000122 0.000450 YES RMS Force 0.000058 0.000300 YES Maximum Displacement 0.000513 0.001800 YES RMS Displacement 0.000296 0.001200 YES |
|
Frequency
Frequency file: here
| summary data | low modes |
|---|---|

|
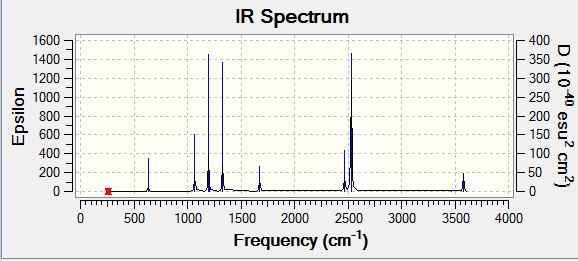
Low frequencies --- -0.0011 -0.0004 0.0009 17.3039 18.1059 37.5201 Low frequencies --- 265.8630 632.2227 639.3730 |
Vibrational spectrum for NH3BH3
| wavenumber | Intensity | IR active? | type |
| 265.86 | 0 | no | bend |
| 632.22 | 14.0296 | slight | stretch |
| 639.37 | 3.5492 | no | bend |
| 639.45 | 3.5493 | no | bend |
| 1069.35 | 40.5084 | yes | bend |
| 1069.39 | 40.5106 | yes | bend |
| 1196.51 | 109.0442 | yes | stretch |
| 1203.78 | 3.4965 | no | bend |
| 1203.81 | 3.4978 | no | bend |
| 1329.38 | 113.5364 | yes | stretch |
| 1676.24 | 27.5506 | yes | bend |
| 1676.24 | 27.5522 | yes | bend |
| 2470.37 | 67.2128 | yes | stretch |
| 2530.29 | 231.3323 | yes | stretch |
| 2530.31 | 231.3222 | yes | stretch |
| 3462.65 | 2.5089 | no | stretch |
| 3759.59 | 27.9221 | yes | stretch |
| 3759.61 | 27.9227 | yes | stretch |
Determination of Bond Energy
E(NH3)=-56.55776873 a.u
E(BH3)=-26.46226371 a.u
E(NH3BH3)=-83.22468893 a.u
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
=-83.22468898-(-56.5577687-26.46226371) =-0.20465657 a.u
1 a.u=2625.50 kj/mol
ΔE=-537.3258245 kj/mol
Aromaticity
All aromatic molecules were optimised using the full basis set 6-31G(d,p).
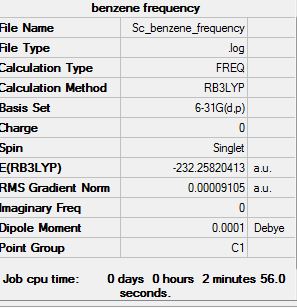
Benzene
Optimisation
Optimisation log file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|

|
Item Value Threshold Converged?
Maximum Force 0.000198 0.000450 YES
RMS Force 0.000082 0.000300 YES
Maximum Displacement 0.000849 0.001800 YES
RMS Displacement 0.000305 0.001200 YES
|
|
Frequency Analysis
Frequency file: here
| summary data | low modes |
|---|---|
|
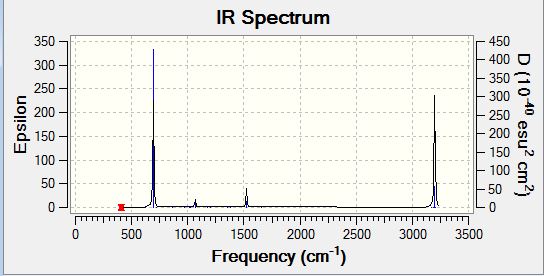
Low frequencies --- -13.7810 -12.9763 -11.9029 -0.0009 -0.0009 -0.0002 Low frequencies --- 414.0699 414.1914 620.9703 |
Vibrational spectrum
| wavenumber | Intensity | IR active? | type |
| 414.07 | 0 | no | bend |
| 414.19 | 0 | no | bend |
| 620.97 | 0 | no | bend |
| 620.99 | 0 | no | stretch |
| 693.20 | 74.2469 | yes | bend |
| 718.29 | 0 | no | bend |
| 864.24 | 0 | no | bend |
| 864.27 | 0 | no | bend |
| 973.74 | 0 | no | bend |
| 973.81 | 0 | no | bend |
| 1012.46 | 0 | no | bend |
| 1017.87 | 0 | no | bend |
| 1019.92 | 0 | no | bend |
| 1066.39 | 3.4013 | no | bend |
| 1066.45 | 3.4005 | no | bend |
| 1179.26 | 0 | no | bend |
| 1202.22 | 0 | no | bend |
| 1202.23 | 0 | no | bend |
| 1356.07 | 0 | no | bend |
| 1380.24 | 0 | no | bend |
| 1524.39 | 6.6149 | no | bend |
| 1524.44 | 6.6185 | no | bend |
| 1653.03 | 0 | no | bend |
| 1653.14 | 0 | no | bend |
| 3174.37 | 0.0004 | no | stretch |
| 3183.88 | 0.0001 | no | stretch |
| 3183.98 | 0.0001 | no | stretch |
| 3199.54 | 46.5648 | yes | stretch |
| 3199.65 | 46.5377 | yes | stretch |
| 3210.18 | 0.0003 | no | stretch |
Benzene MO Diagram
NBO analysis
Optimised file: here
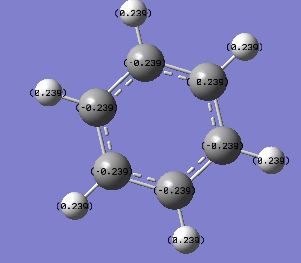
As benzene is a symmetrical molecule, it has a symmetrical charge distribution. All carbon atoms have -0.239 charges whereas hydrogen atoms have +0.239 charge. Carbon atoms has negative charges as they are more electronegative than hydrogen atoms; pulling electron towards itself. All charges add up to zero to give overall zero charge. The dipole moment is very close nearly zero due to symmetrical charge distribution.
Optimisation Comparison
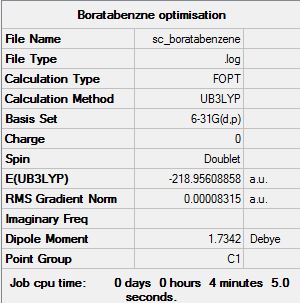
Boratabenzne:B3LYP/6-31G(d,p)level
Optimisation log file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|
|
Item Value Threshold Converged? Maximum Force 0.000152 0.000450 YES RMS Force 0.000043 0.000300 YES Maximum Displacement 0.000704 0.001800 YES RMS Displacement 0.000172 0.001200 YES |
|
Boratabenzne contains one "B-H" replacing of "C-H". An extra charge needs to be added to be isoelectronic as B is more electropositive than C. Therefore the energy of the molecule is slightly higher than benzene and the dipole moment is different to that of benzene.
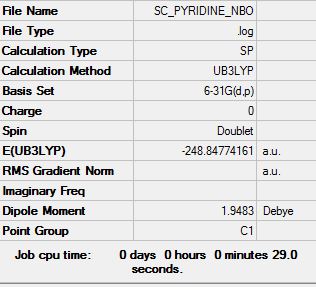
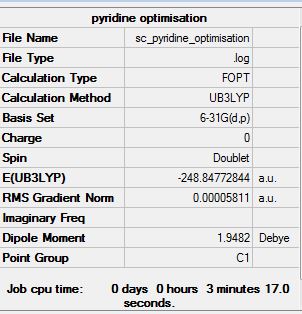
pyridine:B3LYP/6-31G(d,p)level
Optimisation log file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|
|
Item Value Threshold Converged? Maximum Force 0.000157 0.000450 YES RMS Force 0.000038 0.000300 YES Maximum Displacement 0.000859 0.001800 YES RMS Displacement 0.000210 0.001200 YES |
|
Pyridine contains one "N-H" replacing of "C-H". An extra charge needs to be removed to be isoelectronic as N is more electronegative than C. Therefore the energy of the molecule is slightly lower than benzene and the dipole moment is different to that of benzene.
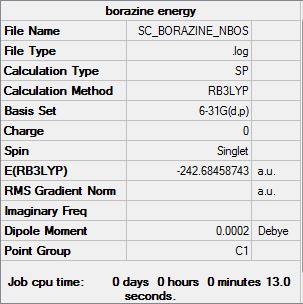
Borazine:B3LYP/6-31G(d,p)level
Optimisation log file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|
|
Item Value Threshold Converged? Maximum Force 0.000085 0.000450 YES RMS Force 0.000033 0.000300 YES Maximum Displacement 0.000249 0.001800 YES RMS Displacement 0.000077 0.001200 YES |
|
Borazine is aromatic compound with NH and BH units alternating. As it is symmetric molecule, it is isostructural and isoelectric to benzene. Therefore the dipole moment is similar to benzene; close to zero.
Frequency Comparison
Boratabenzene:B3LYP/6-31G(d,p)
Frequency file: here
| summary data | low modes |
|---|---|
|
Low frequencies --- -7.2032 -0.0009 0.0003 0.0004 1.9815 3.1157 Low frequencies --- 245.4562 344.7212 500.5873 |
Pyridine:B3LYP/6-31G(d,p)
Frequency file: here
| summary data | low modes |
|---|---|

|
Low frequencies --- -252.1317 -9.0082 -7.6210 -0.0008 -0.0007 0.0004 Low frequencies --- 3.6141 242.7426 437.0176 |
Borazine:B3LYP/6-31G(d,p)
Frequency file: here
| summary data | low modes |
|---|---|
|
Low frequencies --- -3.9223 -0.0011 -0.0010 -0.0008 7.4410 9.4491 Low frequencies --- 289.5787 289.7159 404.3426 |
NBO Comparison
Boratabenzene
Optimised file: here
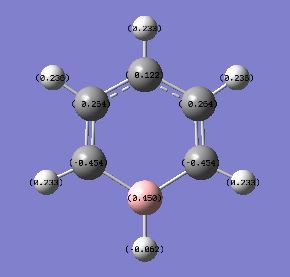
The Boron atom has the charge of 0.450 indicating that Boron is electropositive atom. This makes the adjacent carbon atoms electronegative; withdrawing electrons towards itself. Therefore the charge distribution is unsymmetrical. The dipole moment is 1.7338 due to the presence of electropositive boron atom.
Pyridine
Optimised file: here
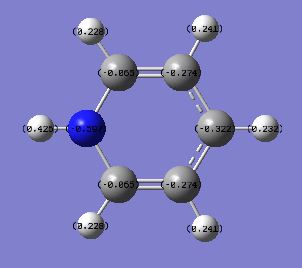
The Nitrogen atom has the charge of -0.597 indicating that nitrogen is electronegative atom; withdrawing electrons towards itself. The presence of Nitrogen distorts the structure of the molecule, therefore the charge distribution is unsymmetrical. The dipole moment is 1.9483 due to the presence of electronegative nitrogen atom.
Borazine
Optimised file: here
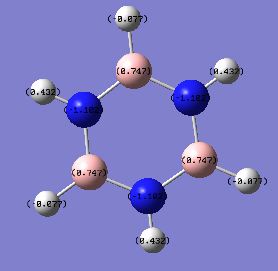
The B-H unit and N-H unit are alternating in Borazine molecule which makes the molecule symmetrical; all Nitrogen atoms have the same charges of -1.102 indicating that nitrogen is electronegative atom whereas all Boron atoms have the charge of +0.747. The dipole moment is close to zero due to symmetrical charge distribution.
MO Comparison
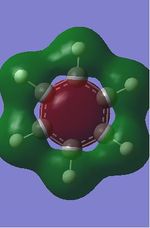
| Benzene | Boratabenzene | Pyridine | Borazine | ChemDraw diagram | |
|---|---|---|---|---|---|
| 1. | 
|
|

|

|
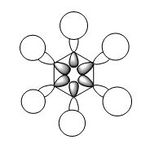
|
| Energy(au) | -0.51795 | -0.50648 | -0.55092 | -0.55132 | - |
| MO placement | 12 | 12 | 12 | 10 | - |
| 2. | 
|
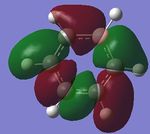
|

|
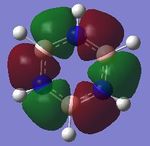
|

|
| Energy(au) | -0.43854 | -0.42598 | -0.45916 | -0.43197 | - |
| MO placement | 14 | 15 | 14 | 15 | - |
| 3. | 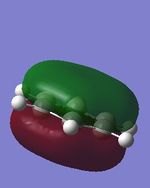
|
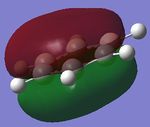
|
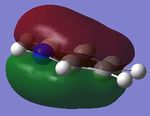
|

|
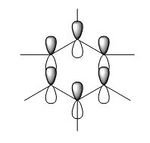
|
| Energy(au) | -0.35999 | -0.36130 | -0.39718 | -0.36129 | - |
| MO placement | 17 | 17 | 17 | 17 | - |
The first set of MOs show the overlaps of s orbitals of hydrogen atoms and p orbitals of carbon, boron and nitrogen atoms. The phase difference between hydrogen atoms and central atoms represents highly bonding character. The MO energies of benzene and boratabenzene are similar to each other as electronegativity of boron is similar to electronegaivity of carbon. However the MO energies of pyridine and borazine are much lower than benzene and boratabenzene due to highly electronegative Nitrogen. The pyridine MO show the nitrogen atom polarise the molecule towards itself therefore the shapes of the MO are slightly distorted. The Borazine MO shows this more significantly due to more nitrogen atoms are present.
The second set of MOs show the sigma overlaps of p orbitals of central atoms. The MO energies of benzene and borazine are similar to each other as their structures are highly symmetric. The MOs of boratabenzene and pyridine are quite distorted due to presence of one B-H unit and N-H unit consecutively. The MO energy of pyridine is much more lower than the others due to the presence of electronegative N atom and this pulls the electrons towards itself making the MO more distorted.
The last set of MOs show the pi overlaps of p orbitals of central atoms. The phase differences between above and below the plane with no nodes show high bonding character. Just like other sets of MOs, pyridine has the lowest MO energy due to the presence the most electronegative N atom which gives distortion of the molecule as it pulls electrons towards itself.
The full MO diagrams of boratabenzene, pyridine and borazine will look different to each other as they have different substituents with different energies and electronegativities.