Rep:Mod:sk-inorg
Day 1 tasks
BH3
Optimisation & frequency analysis
BH3 was optimised using the B3LYP method and the 6-31G(d,p) basis set. Below is:
- The summary window, which shows the correct molecular symmetry (D3h);
- The item table in the log file obtained after the optimisation, which shows that the optimisation is fully completed;
- The low frequencies obtained from the BH3 frequency log file.
Item Value Threshold Converged?
Maximum Force 0.000012 0.000450 YES
RMS Force 0.000008 0.000300 YES
Maximum Displacement 0.000064 0.001800 YES
RMS Displacement 0.000039 0.001200 YES
Predicted change in Energy=-1.128858D-09
Optimization completed.
-- Stationary point found.
Low frequencies --- -12.3492 -12.3425 -7.6649 -0.0008 0.0239 0.4061 Low frequencies --- 1162.9695 1213.1356 1213.1358
Optimised BH3 molecule |
Vibrational modes
| Wavenumber (cm-1) | Intensity (arbitrary units) | Symmetry | IR active? | Vibrational mode |
|---|---|---|---|---|
| 1163 | 93 | A2'' | Yes | Out-of-plane bend |
| 1213 | 14 | E' | Very slightly | Symmetric bend |
| 1213 | 14 | E' | Very slightly | Symmetric bend |
| 2583 | 0 | A1' | No | Symmetric stretch |
| 2716 | 126 | E' | Yes | Asymmetric stretch |
| 2716 | 126 | E' | Yes | Asymmetric stretch |
Only three peaks are visible in the IR spectrum (instead of the 6 present in the table given above). The vibration at 2583 cm-1 is not present because this frequency is for the symmetric stretch vibrational mode, which doesn't change the dipole moment of the molecule whereas the other vibrations do. A change in dipole moment is needed for a vibration to be IR active as one of the selection rules for this spectroscopy is Δμ ≠ 0, where Δμ is the change in dipole moment. There is also 2-fold degeneracy present for the vibration at the wavenumbers of 1213 and 2716 cm-1, removing two possible peaks and this means only one peak will be present at these frequencies.
Molecular orbital diagram
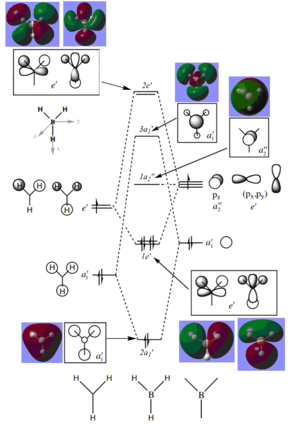
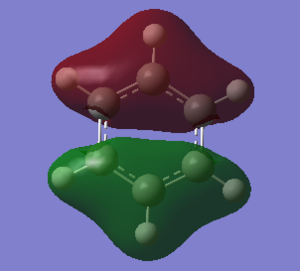
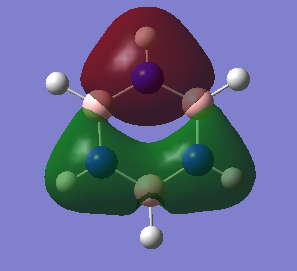
The molecular orbital diagram of BH3 is given with the computed molecular orbitals alongside the predicted LCAOs molecular orbitals.[1]
The 1s orbital of boron (of 1a1' symmetry) was ignored in the diagram as it is too deep in energy to combine with any other hydrogen orbitals. Due to hydrogen being more electronegative than boron:
- The highest energy orbitals of boron, the 2p orbitals, are placed higher up in the diagram than the halfway point of the H3 fragment orbitals
- The bonding orbitals have a larger contribution from the H3 FOs and a smaller contribution from the boron AOs (and vice versa for the antibonding orbitals)
In general, the computed MOs agreed with the predicted ones as the shapes where very similar, showing that qualitative MO theory is useful as it uses relatively simple principles to predict the shapes of the actual MOs, with quite good accuracy; however, there is one significant slight discrepancy: there was a greater contribution from the 1s hydrogen orbitals in the antibonding MOs, especially in the 3a1' MO, where it was predicted that the boron 2s orbital would have a greater contribution due to boron being more electronegative than hydrogen. This shows that qualitative MO theory is not completely accurate as this difference suggests that merely using the electronegativities of boron and hydrogen to create the MOs won't always lead to the correct shapes.
Ng611 (talk) 13:15, 5 June 2018 (BST) Excellent!
NH3
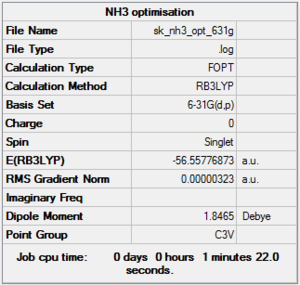
NH3 was optimised using the B3LYP method and the 6-31G(d,p) basis set. Below is:
- The summary window, which shows the correct molecular symmetry (C3v);
- The item table in the log file obtained after the optimisation, which shows that the optimisation is fully completed;
- The low frequencies obtained from the NH3 frequency log file.
Optimisation & Frequency analysis
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000012 0.001800 YES
RMS Displacement 0.000008 0.001200 YES
Predicted change in Energy=-9.843984D-11
Optimization completed.
-- Stationary point found.
Low frequencies --- -0.0138 -0.0032 -0.0015 7.0783 8.0932 8.0937 Low frequencies --- 1089.3840 1693.9368 1693.9368
Optimised NH3 molecule |
NH3BH3
NH3BH3 was optimised using the B3LYP method and the 6-31G(d,p) basis set. Below is:
- The summary window, which shows the correct molecular symmetry (C3v);
- The item table in the log file obtained after the optimisation, which shows that the optimisation is fully completed;
- The low frequencies obtained from the NH3BH3 frequency log file.
Optimisation & Frequency analysis
Item Value Threshold Converged?
Maximum Force 0.000122 0.000450 YES
RMS Force 0.000058 0.000300 YES
Maximum Displacement 0.000531 0.001800 YES
RMS Displacement 0.000296 0.001200 YES
Predicted change in Energy=-1.655851D-07
Optimization completed.
-- Stationary point found.
Low frequencies --- -0.0251 -0.0033 -0.0008 17.1236 17.1258 37.1326 Low frequencies --- 265.7816 632.2034 639.3483
Optimised NH3BH3 molecule |
Association energy of NH3BH3
E(NH3) = -56.55777 a.u.
E(BH3) = -26.61532 a.u.
E(NH3BH3) = -83.22468 a.u.
ΔE = E(NH3BH3)-[E(NH3)+E(BH3)] = -0.05159 a.u. = -135 kJ/mol
Compared to an isolectronic molecule of ethane, with a bond energy of around 376.2 kJ/mol,[2] this value seems quite weak, despite it having a negative change, meaning that this adduct formation is still an exothermic reaction.
BBr3
NH3 was optimised using the RB3LYP method and the GEN basis set. Below is:
- The summary window, which shows the correct molecular symmetry (D3h);
- The item table in the log file obtained after the optimisation (DOI:10042/202460 ), which shows that the optimisation is fully completed;
- The low frequencies obtained from the BBr3 frequency file: DOI:10042/202461
Ng611 (talk) 13:16, 5 June 2018 (BST) You mean LANL2DZ/6-31G -- Gen is not a valid basis set descriptor.
Item Value Threshold Converged?
Maximum Force 0.000008 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000036 0.001800 YES
RMS Displacement 0.000024 0.001200 YES
Predicted change in Energy=-4.085774D-10
Optimization completed.
-- Stationary point found.
Low frequencies --- -2.3055 -0.0029 -0.0018 0.0774 0.7534 0.7534 Low frequencies --- 155.9402 155.9405 267.6894
Optimised BBr3 molecule |
Aromaticity project
Benzene
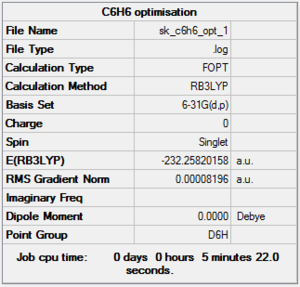
Benzene was optimised using the B3LYP method and the 6-31G(d,p) basis set. Below is:
- The summary window, which shows the correct molecular symmetry (D6h);
- The item table in the log file obtained after the optimisation, which shows that the optimisation is fully completed;
- The low frequencies obtained from the C6H6 frequency log file.
Item Value Threshold Converged?
Maximum Force 0.000193 0.000450 YES
RMS Force 0.000079 0.000300 YES
Maximum Displacement 0.000830 0.001800 YES
RMS Displacement 0.000294 0.001200 YES
Predicted change in Energy=-4.437902D-07
Optimization completed.
-- Stationary point found.
Low frequencies --- -17.2053 -14.9372 -14.9372 -0.0055 -0.0055 -0.0007 Low frequencies --- 414.1053 414.1053 620.9426
Optimised benzene molecule |
Borazine
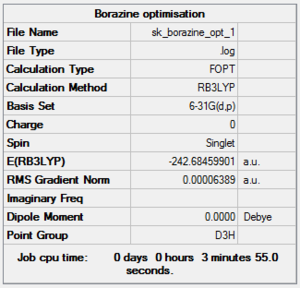
Borazine was optimised using the B3LYP method and the 6-31G(d,p) basis set. Below is:
- The summary window, which shows the correct molecular symmetry (D3h);
- The item table in the log file obtained after the optimisation, which shows that the optimisation is fully completed;
- The low frequencies obtained from the Borazine frequency log file.
Item Value Threshold Converged?
Maximum Force 0.000085 0.000450 YES
RMS Force 0.000033 0.000300 YES
Maximum Displacement 0.000250 0.001800 YES
RMS Displacement 0.000075 0.001200 YES
Predicted change in Energy=-9.233282D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -0.0105 -0.0090 -0.0035 2.8223 2.8805 4.1465 Low frequencies --- 289.7095 289.7101 404.4118
Optimised borazine molecule |
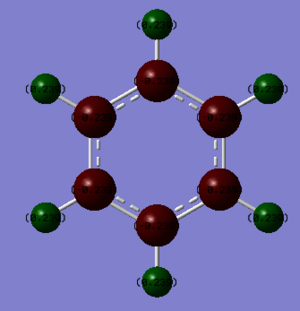
NBO charge analysis
The table below shows the results from the NBO charge analysis of benzene and borazine. In the images below, a colour scale of -1.10 (red) to +1.10 (green) was used and black corresponded to a charge of 0. The charges of each atom in the molecule given below the corresponding image.
| Benzene | Borazine |
|---|---|
|

|
| C = -0.24 | N = -1.10 |
| H = 0.24 | H in NH unit = 0.43 |
| B = 0.75 | |
| H in BH unit = -0.08 |
Being symmetric, both molecules have no dipole moment (all of the charges in each molecule add up to 0), although the charge distribution has symmetry corresponding to the point group of the molecule: all of the symmetry operations in benzene's point group (D6h) can be performed on the charge distribution and it will map onto itself and the same is true for borazine's charge distribution using the symmetry operations in the D3h point group. The C-H bonds in benzene are only very slightly polarised, with the carbon atoms being red as it's slightly more electronegative than hydrogen. Borazine's charge distribution is more varied because of the alternating nitrogen/boron pattern in the ring. The nitrogen atoms are bright red because they are the most electronegative atoms present and the boron atoms are bright green as, even though they have a similar electronegativity to the hydrogen atoms, each boron is between two nitrogen atoms, so it will have a more positive charge compared to the hydrogen atoms. The Hydrogen atoms in the NH units have a greater positive charge than the H atoms in the BH units due to being bonded to a more electronegative atom (nitrogen), so more electron density is removed from H than when bonded to B.
Ng611 (talk) 13:21, 5 June 2018 (BST) A thorough but efficient discussion -- well done. Another interesting point to note is that the total partial charge value of each B-H/N-H pair is identical.
Comparison of benzene and borazine MOs
In the following discussion, the z-axis is taken to be the principal axis of each molecule.
Ng611 (talk) 13:26, 5 June 2018 (BST) Good comparisons made here. Some other points to consider would be the character (sigma, pi, etc.) of the orbital and also their symmetry labels.
Aromaticity
Aromaticity can be explained simply using Hückel's rules, where an aromatic molecule fulfils these rules:
- It must be planar,
- Cyclic,
- Have a contiguous array of p orbitals orthogonal to the plane of the ring
- Have 4n+2 electrons in these p orbitals, where n is an integer
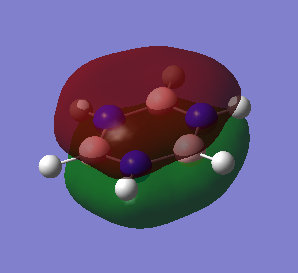
Benzene and borazine both comply with these rules as they're both cyclic and planar, each with 6 electrons in the pz orbitals (each carbon contributes 1 electron in benzene; in borazine, each B contributes no electrons and each N has 2 electrons in the pz orbitals) and MO 17 for both shows the contiguous array of pz orbitals.
It is known that there is a "resonance energy" associated with aromatic compounds, which makes them very stable compounds.[3] In an applied magnetic field, the ring current in aromatic systems creates its own induced magnetic field. The effect of this is that outside the ring, the induced field is in the same direction and the applied field, so hydrogens outside the ring would be deshielded in an NMR spectrometer. The opposite is true inside the ring.
It has been suggested that the sigma framework in aromatic molecules (the p orbitals combining in-phase in the plane of the ring) is an important bonding mode as well, not solely the pz orbitals.[4]
Ng611 (talk) 13:27, 5 June 2018 (BST) I would suggest adding more detail/exposition in this section, although the points that you've made are all correct and accurate.
Ng611 (talk) 13:28, 5 June 2018 (BST) An excellent report overall, well done. Besides some missing detail in one or two discussions, there is very little to criticize here. Very well done.
- ↑ 1.0 1.1 http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf [Accessed 23/05/2018]
- ↑ Yu-Ran Luo and Jin-Pei Cheng "Bond Dissociation Energies" in CRC Handbook of Chemistry and Physics, 96th Edition
- ↑ T. M. Krygowski, M. K. CyraÇski, G. Haefelinger, A. R. Katritzky, Tetrahedron 2000, 56, 1783.
- ↑ M. Palusiak and T. M.Krygowski, Chem. Eur. J., 2007, 13, pp. 7996-8006