Rep:Mod:shaneomar
Module 1
The Hydrogenation of Cyclopentadiene Dimer

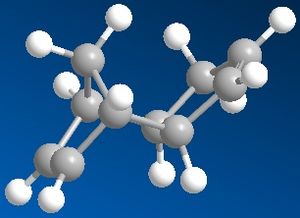
In order to investigate the dimerisation of Cyclopentadiene, the relative energies of the two possible products were calculated using molecular modelling techniques (Chem3D). The endo dimer is known to be produced specifically over the exo dimer. However, the results of molecular modelling show that the endo dimer is thermodynamically less stable by 2.14 kcal/mol (34.02-31.88). This suggests that the cyclodimerisation of cyclopentadiene is a kinetically controlled process, since the product which is more strained/hindered is specifically formed. An explanation for this would be that the transition state on forming the endo dimer is of lower energy than that of the exo product.
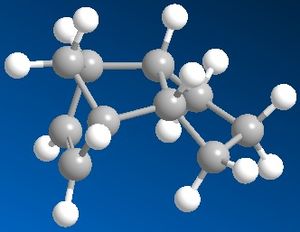
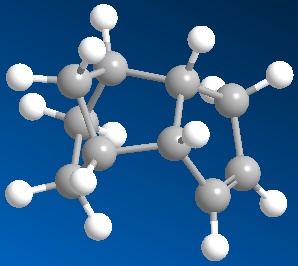
The hydrogenation of the cyclopentadiene molecule forms one of two dihydro products initially, then upon further hydrogentation forms the tetrahydro derivative. By performing molecular modelling analysis of these two dihydro products, it can be determined which of the two double bonds (of the endo dimer) will be hydrogenated first.
According to the results, Dihydro derivative b (pictured on the right) is of lower energy by 4.55 kcal/mol (35.70-31.15). This suggests that the dihydro derivative formed on hydrogenating the double bond in the bridged ring is more thermodynamically stable. Therefore it is likely that this is the first double bond to undergo hydrogenation, because it is the most easily hydrogenated thermodynamically. This is also supported by the experimental relative activation energies of each hydrogenation step[1].
To understand why hydrogenating this double bond is more favourable than the other, the relative contributions from stretching, bending, torsion, van der Waals and hydrogen bonding were analysed. These values are mostly similar in both dihydro derivatives, except for the contribution from bending - which is by far the largest difference between the derivatives. The contribution to overall energy from bending is 5.30 kcal/mol larger in dihydro derivative a than that in derivative b (19.81-14.51). A larger contribution from bending could be rationalised by looking at the C=C-C bond angles in each molecule. Derivative a has a C=C-C angle of 103.6o, whilst the angle of the C=C-C in derivative b is 113.0o. The fact that this angle around the double bond in the bridged ring (derivative a) is significantly smaller explains why the molecule has a larger contribution to energy from bending, hence why the overall energy is higher than that of derivative b.
Stereochemistry of Nucleophilic additions to pyridiniun ring (NAD+ analogue)
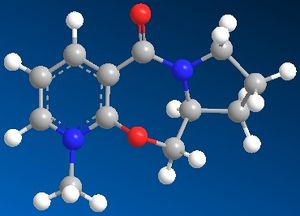
The methylation reaction between a Grignard reagent (methyl magnesium iodide) and the pyridine ring in the following compound 5 was investigated using molecular mechanics and also the MOPAC/PM6 molecular orbital method.
When using the molecular mechanics for compound 5, only one minimum of energy could be found when different starting conformations were tried (N+ has been reported to cause a bug in the molecular modelling). However, using MOPAC/PM6, another minimum energy conformation could be found. Once the energy was minimised, the geometry of the molecules were explored by looking at the dihedral angle between the pyridine ring and the carbonyl group. This angle was found to be close to zero when using molecular mechanics, but with MOPAC the another angle was found at a minimum of energy (reported as 93.68 kcal/mol) which was 28.2o.
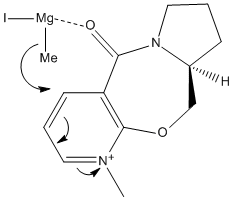
The reaction delivers the methyl group selectively with stereochemistry which leaves the group pointing in the same direction as the carbonyl group was angled towards. This can be explained by drawing the mechanism of the reaction (below) which shows the coordination[2] between the magnesium atom and the lone pair on the carbonyl oxygen:
This interaction leads to the formation of mainly one stereoisomer, that which would have the methyl group pointing towards you on the diagram shown. The direction the carbonyl group is pointing dictates the side side the methyl group gets added to. This is becasue once the coordination between the Grignard reagent and the carbonyl oxygen occurs, the orientation of the methyl group is such that it can only add to the same side of the molecule.
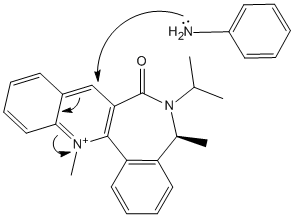
Compound 7 (shown on the right) reacts with aniline which adds an NHPh group to the pyridine ring. By using the PM6 method in MOPAC, the energy of compound 7 was minimised and different starting conformations were tried to find the lowest energy possible (reported as 157.44 kcal/mol). This structure shows that the carbonyl group is facing the opposite way compared to compound 5, this time at a dihedral angle between it and the pyridine ring of 45.1o.
The NHPh group adds to the pyridine ring on the opposite face of the molecule than the one the carbonyl group is pointing out from. This can be explained by the steric hindrance caused by the oxygen of the carbonyl group blocking the large aniline group from attacking on this side. Whichever conformation was tried in the calculation, the carbonyl group could only face in one direction unless forced into a very unstable position (high energy). This shows that the attacking aniline group will very rarely be able to approach from the other side. Therefore the product is formed with the same stereochemistry as the previous reaction (involving compound 5 and Grignard) despite the carbonyl groups of each reactant compound 5 and 7 pointing in opposite directions.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
The stereochemistry of the carbonyl group in the following molecule was investigated in order to find out which isomer was the more stable. The calculations were carried out using the MM2 force field and the energy of each molecule was minimised:
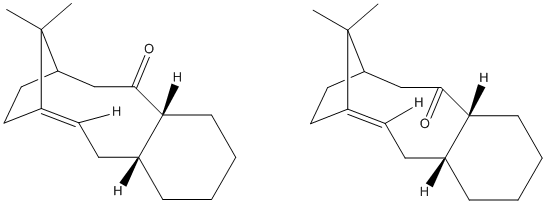
Initial energy values were reported for compound 10 (carbonyl 'down') of around 46 kcal/mol. Upon trial of new starting conformations (such as boat conformer ring) a minimised energy was calculated as 44.29 kcal/mol. Applying the same process to compound 9 (carbonyl 'up'), the lowest minimised energy was found to be 59.73 kcal/mol. These values show that the isomer 10 with the carbonyl 'down' was the more thermodynamically stable.
An explanation for compound 10 being more stable can be found by looking at the contributions to energy in the output. The values are mostly similar except with the bend contribution to energy, which is 10.7 kcal/mol with the carbonyl 'down' and 20.8 kcal/mol with the carbonyl 'up'. The increased energy from bending is likely due to the angle at which the C-CO-C carbons around the carbonyl group in each isomer. In compound 9 that angle is close to the ideal 120o at 120.3o, whereas this angle in compound 9 is 127.7o.
This molecule is has an example of a 'hyperstable alkene'[3] and the alkene group reacts very slowly. This can be investigated by considering the type of product of the reaction upon losing the alkene group. The model was modified to produce a hydrogenated version (no alkene group). After minimising the energy of hydrogenated compound 10 in the MM2 force field, the energy was reported as 53 kcal/mol, and for hydrogenated compound 9 it was 66 kcal/mol. However, this does not explain why the alkene in compound 10 reacts so slowly. What is more useful is to look at the bond angles of the new sp3 carbon atoms. These show significant deviation from the ideal 109.5o, which is due to the presence of the adjacent ring. Also, the extra group added to the molecule (H or Br etc) will add bulk to the groups in the molecule and so the products would be more unstable than the alkene. Therefore the alkene, despite not being very stable itself, will react slowly due to the likely high activation energy of reaction.
The MMFF94 gave values for the two isomers 10 and 9 as 60.5 kcal/mol and 82.7 kcal/mol respectively. This shows the same result as with MM2, which is that compound 10 is the more stable isomer.
Regioselective Addition of dichlorocarbene
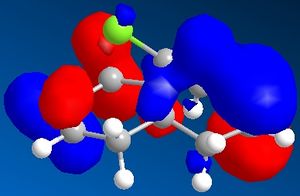
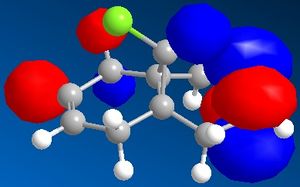
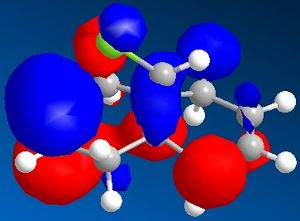


The minimised energy and geometry of the molecule 12 was calculated first using the MM2 field in molecular modelling, then using MOPAC/PM6. The minimum energy using MM2 was found to be 17.90 kcal/mol and using MOPAC/PM6 was 19.74 kcal/mol. The molecular surfaces were also generated in Chem3D and the pictures show the HOMO-1, HOMO, LUMO, LUMO+1 and LUMO+2 molecular orbital surfaces.
The HOMO picture clearly shows that the double bond exo to the chlorine has much less electron density. Because this double bond has a much smaller HOMO surface, it must be less reactive as a nucleophile (such as dichlorocarbene). Therefore you would expect the other double bond to be more reactive.
Vibrational data could not be found even after following instructions...
Structure based Mini Project: Lentiginosine epimers
Ring saturated nitrogen cycles are known to be components of ant venom and amphibian skin. Polyhydrated indolizidine alkaloids such as (+)-lentiginosine and its isomers are known to have pharmocological potential as amyloglycosidase inhibitors. The following two compounds are the stereoisomeric products formed in a concise synthesis which uses the Mitsunobu reaction, in which the NMR data is also reported[4].
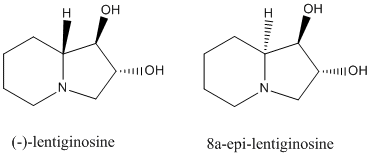

One of the final steps in this reaction involves PtO2-catalyzed diastereoselective hydrogenation of a pyridinium ring. The result is that (-)-lentiginosine and it's epimer 8a-epi-lentiginosine are formed in a ratio 0.8:1, which means the reaction is only slightly diastereoselective towards 8a-epi-lentiginosine.
To firstly analyse whether molecular modelling could rationalise the preference described above, the compounds were built and their energy minimised using the MM2 force field in Chem3D. The results showed that the overall energy of 8a-epi-lentiginosine is 0.67 kcal/mol lower in energy than the other isomer (22.80-22.13 kcal/mol). This confirms the slight stereoselectivity of the reaction in favour of the 8a-epi-lentiginosine. The reason for the preference is due to loss of repulsion when the hydroxyl group is anti (instead of syn) to the hydrogen in question (there is also less ring strain when this is the case). One way to increase the yield of 8a-epi-lentiginosine could therefore be to use a bulky protecting group on this alcohol to increase the repulsion in the alternative product.
The carbon-13 NMR (CDCl3, TMS) data for both molecules were calculated using the GIAO appraoch. The results are shown below[5][6]:
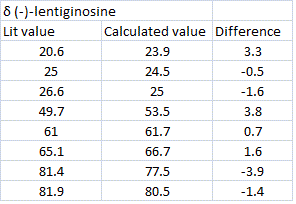
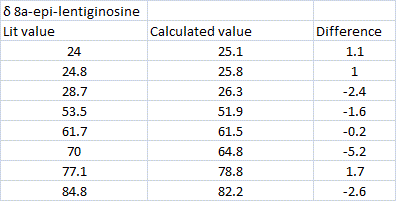
The calculated values for chemical shift are a good match to the literature. The only carbon which has a chemical shift more than 5ppm difference is the one at 64.8. This is the carbon which changes it's stereochemistry between the two epimers. Since the value is more than 5ppm lower than the literature this suggests the experimental chemical shift for this carbon may have been incorrectly assigned or a technical inaccuracy. The two molecules' carbon-13 NMR spectra are difficult to distinguish between since the chemical shifts are very similar. Therefore, in order to check whether the two assignments are correct, the reported 1H NMR coupling constants could be compared to the calculated dihedral angles (Karplus equation).
References
- ↑ http://www.vurup.sk/pc/vol45_2003/issue3-4/pdf/05.pdf - Kinetics of Dicyclopentadiene Hydrogenation
- ↑ DOI:10.1021/jo00356a016
- ↑ Hyperstable olefins - DOI:10.1021/ja00274a016
- ↑ Concise synthesis of lentiginosine deriviatives - DOI:10.1021/jo702141b
- ↑ (-)-lentiginosine NMR - DOI:10042/to-5696
- ↑ 8a-epi-lentiginosine NMR - DOI:10042/to-5699
