Rep:Mod:sh7118
NH3
Information from running an optimisation
Calculation Method RB3LYP Basis Set 6-31G(d,p) E(RB3LYP) -56.44397188 RMS Gradient Norm 0.05399560 Point Group C3V
N-H bond length: 1.30000
H-N-H bond angle= 109.471
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Jmol image of NH3
test molecule |
File:SHUSSAIN NH3 OPTF POP.LOG
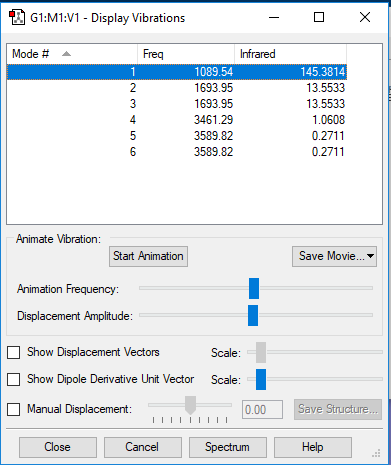
Frequency analysis
Vibration 1 2 3 4 5 6 Wavenumber cm-1 1090 1694 1694 3461 3590 3590 IR Intensity 145.4 13.55 13.55 1.062 0.2711 0.2711 Symmetry A1 E E A1 E E
Q1) (4x4)-6=6 modes
Q2) Modes that have same frequency are degenerate so mode that has 1694(2 and 3) and mode that has 3590( 5 and 6)
Q3) Degenerates modes are bending so modes that has 1694(2 and 3) and mode that has 3590( 5 and 6). Mode with frequency 1090 (1) and 3461(4) bond stretch vibrations.
Q4) Mode that has 3461 frequency is symmetric
Q5) Mode that has 1090 frequency is known as the "umbrella" mode
Q6) two bands would be expected as vibrations that has frequency of 3461,3590 has low intensity. There are three different vibration with high intensity but here is a degenerate at frequency of 1694. Two bands would be shown at 1090 and 1694.
Charge analysis
Nitrogen= -1.125 and Hydrogen= 0.375
I would expect Nitrogen to be negative and Hydrogen to be positive because nitrogen is more electronegative than hydrogen so it will draw electron density towards itself.
N2
Information from running an optimisation
Calculation Method RB3LYP Basis Set 6-31G(d,p) E(RB3LYP) -109.52359111 RMS Gradient Norm 0.02473091 Point Group D*H
N-N bond length=1.09200
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Jmol image of N2
test molecule |
File:SHUSSAIN N2 OPTIMISATIONN POP.LOG
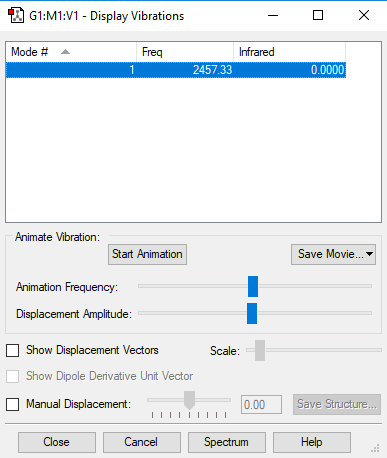
Frequency analysis
Vibration 1 Frequencies cm-1 2457 IR Intensity 0 Symmetry SGG
Charge analysis
Both nitrogen has zero charge as expected as it a is non-polar molecule.
H2
Information from running an optimisation
Calculation Method RB3LYP Basis Set 6-31G(d,p) E(RB3LYP) -1.15928020 RMS Gradient Norm 0.09719500 Point Group D*H
H-H bond length=0.6000
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Jmol image of H2
test molecule |
File:SHUSSAIN H2 OPTIMISATION POP.LOG
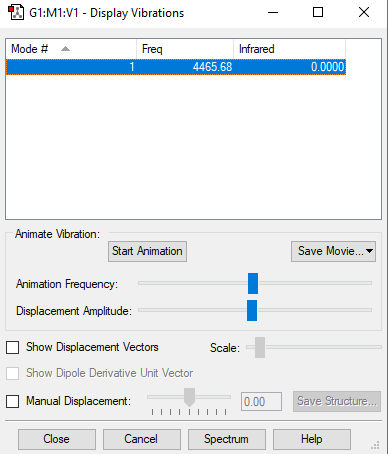
Frequency analysis
Vibration 1 Frequencies 4466 IR Intensity 0 Symmetry SGG
Charge analysis
Both Hydrogen has zero charge as expected as it is a non-polar molecule.
Haber-Bosch process
AQEZIH= mono metal TM complex that coordinates N2. N-N bond length of 1.127(2). Bond lengths are different because N2 is attached to metal complex so electron density of one nitrogen is withdrawn to metal where as in nitrogen molecules, it is a covalent molecule with equal distribution of electron density.
E(NH3)= -56.55776873, 2*E(NH3)= -113.1155375, E(N2)= -109.52412868, E(H2)= -1.17853936, 3*E(H2)= -3.53561808,
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= (-113.1155375)-(-109.52412868-3.53561808)= -0.05579074 au= -146.5 kj/mol
It is an exothermic reaction so products are more stable than reactants so therefore ammonia is more stable.
Choosen Molecule NF3
Information from running an optimisation
Calculation Method RB3LYP Basis Set 6-31G(d,p) E(RB3LYP) -354.03254435 RMS Gradient Norm 0.07304504 Point Group C3V
N-F Bond length: 1.28000 F-N-F Bond angle: 109.471
Item Value Threshold Converged? Maximum Force 0.000164 0.000450 YES RMS Force 0.000108 0.000300 YES Maximum Displacement 0.000612 0.001800 YES RMS Displacement 0.000296 0.001200 YES
Jmol image of NF3
test molecule |
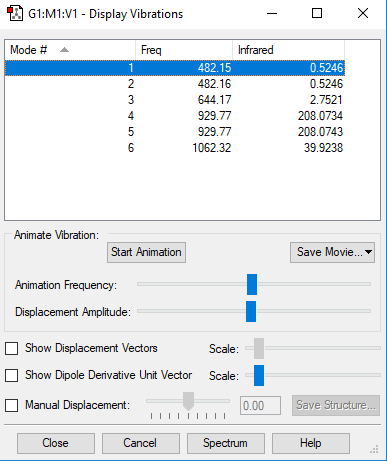
Frequency analysis
Vibration 1 2 3 4 5 6 Wavenumber cm-1 482.2 482.2 644.2 929.8 929.8 1062 IR Intensity 0.5246 0.5246 2.752 208.1 208.1 39.92 Symmetry E E A1 E E A1
Charge analysis
Nitrogen has charge of 0.660 and Fluorine has charge of -0.220 and this is expected as fluorine is more electromotive than nitrogen.
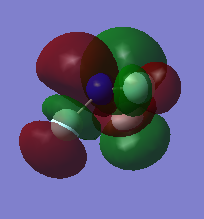
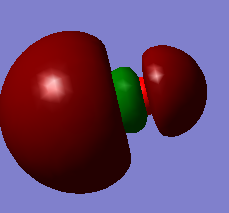
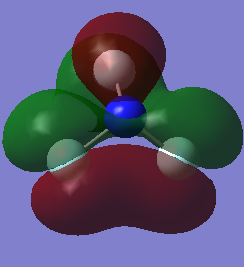
MO of NF2
LUMO which is not occupied by electrons.It is anti bonding orbital. 2px and 3px are involved. It has no effect on bonding but its involved when electron makes transition from HOMO to this orbital usually in spectroscopy.
HOMO which is occupied by electrons and this is bonding orbital. 3s and 2pz are involved.
1s atomic orbitals are involved. MO is deep in energy which is occupied by electrons. It is non bonding.
2py atomic orbital is involved which forms pi molecular orbital. it is occupied by electrons and is bonding orbital.
3px atomic orbital is involved to form pi molecular orbital. It is occupied by electrons and is bonding orbital. It is degenerate to molecular orbital formed by 2py because of the symmetry.
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 0.5/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES - however you have used the default caption of "test molecule" for each of your jmols, this does not help the reader.
NH3 1/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES
N2 and H2 0.5/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES
Crystal structure comparison 0/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
No, you included the unique identifier but didn't reference properly with a direct link.
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 3.5/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES - good explanation of charges well done! Your analysis of the MOs is rather sparse and incorrect in places. It could have been improved by explaining which atoms the AOs are contributing from. The HOMO is antibonding between the F atoms and the N atom - nodal planes intersect the F-N bonds in this MO.
Independence 0/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or Do some deeper analysis on your results so far