Rep:Mod:sh5214 transition states
Introduction
In a potential energy surface, the minimum can either represent stable chemical compounds or it can represent the equilibrium positions between 2 or more compounds in a system. The transition state is the point with the maximum amount of energy going from one minimum to the other. The gradient for both of the points mentioned above is zero, however the curvature for a minimum is a minimum, whereas the curvature for a transition state is a saddle point.
Nf710 (talk) 18:24, 21 March 2017 (UTC) This is a very basic definition. And only in 1 dimension. You should be talking in 3N-5 dimensions. and defining the curvature with the second derivatives
Exercise 1
MO diagram of the TS
Note: MO 16 & 17 are not degenerate, and MO 18 & 19 are not degenerate.
(Fv611 (talk) 17:23, 15 March 2017 (UTC) You should have drawn the fragment orbitals on your diagram. However, well done on getting the relative energies spot on!)
From the MO diagram above it can be seen that the orbitals only interact with another orbital with the same symmetry, this mean that for a reaction to occur, the orbitals interacting must have the same symmetry. This means that the orbital overlap integral for symmetric-symmetric and antisymmetric-antisymmetric interactions are non-zero, and that the the orbital overlap integral for symmetric-antisymmetric interactions are zero.
| MO of Butadiene | |||||
|---|---|---|---|---|---|
|
|
| ||||
| MO of Ethene | |||||
|
|
| ||||
| MO of TS | |||||
|
|
| ||||
|
|
| ||||
Bonds Lengths
| Reactant (Å) | TS (Å) | Product (Å) | |
|---|---|---|---|
| Bond 1 | 1.33530 | 1.37975 | 1.50034 |
| Bond 2 | 1.46835 | 1.41108 | 1.33766 |
| Bond 3 | 1.3530 | 1.37976 | 1.50034 |
| Bond 4 | 1.32731 | 1.38174 | 1.54076 |
| Bond 5 | n/a | 2.11459 | 1.54003 |
| Bond 6 | n/a | 2.11490 | 1.54004 |
As the reaction progresses, the two double bonds on butadiene and the double bond on ethene increases in length, and the single bond on butadiene decreases in length as we form the transition state. This trend continues as we go from the transition state to the product. The typical length of a sp3 C-C bond is 1.54 Å, the typical length of a sp2 C-C bond is 1.47 Å and the typical bond length of a alkene double bond is 1.34 Å. The Van der Waals radius of C atom is 1.7 Å, this is shorter than the partially formed bonds in the TS which had a length of around 2.11 Å.
(Fv611 (talk) 17:23, 15 March 2017 (UTC) If the VdW radius of Carbon atoms is 1.7, the minimum distance of two non-interacting Carbon atoms is 3.4, which is larger than 2.11.)
Vibration that corresponds to the reaction pathway of the TS (synchronous )
From the vibration above it can be seen that the formation of the two bonds is synchronous.
Exercise 2
MO diagram of the exo TS
From my calculations, the results showed that the energy level ordering in the MO diagram for both the exo and the endo diels-alder reaction are the same. My MO diagram also shows that the LUMO of the 1,3-dioxole has a higher energy than the LUMO of cyclohexadiene. This means that in this reaction, the electron rich species is the dienophile and the electron deficient species is the diene, meaning that the reaction is an inverse demand diels-alder reaction. This result is expected due to the fact that the dienophile, 1,3-dioxole, has electron donating O atoms, hence it is electron rich.
| MO of 1,3 dioxole | |||||
|---|---|---|---|---|---|
|
|
| ||||
| MO of cyclodexadiene | |||||
|
|
| ||||
| MO of exo TS | |||||
|
|
| ||||
|
|
| ||||
| MO of endo TS | |||||
|
|
| ||||
|
|
| ||||
Thermochemistry
| Cyclohexadiene (kJ/mol) | 1,3-dioxole (kJ/mol) | TS (kJ/mol) | Product (kJ/mol) | Reaction barrier (kJ/mol) | Reaction energy (kJ/mol) | |
|---|---|---|---|---|---|---|
| Exo | -612616.977634 | -701203.362889 | -1313662.70287 | -1313891.64648 | 157.637658 | -71.30595993 |
| Endo | -612616.977634 | -701203.362889 | -1313670.41921 | -1313895.1594 | 149.921312 | -74.8188792 |
From the table above, it can be seen that the reaction barrier for the endo product is lower than that for the exo product, thus the endo product is the kinetically favoured product. We can also see that the reaction energy of the endo product is more negative than that of the exo product, thus the endo product is also the thermodynamically favoured product.
From the TS MOs above, it can be seen that in the HOMO of the exo TS only one set of orbitals interact, whereas in the HOMO of the endo TS there are two sets of orbitals interacting. This means that the endo TS is more stable than the exo TS, and therefore the reaction barrier for the endo diels-alder reaction is lower than that for the exo diels-alder reaction.
19:29, 21 March 2017 (UTC) You have got the correct energy for the product and the Transition state but your reactants are slightly out and hence your answer is slightly out. You however come to the correct conclusion. But with minimal explanation.
Exercise 3
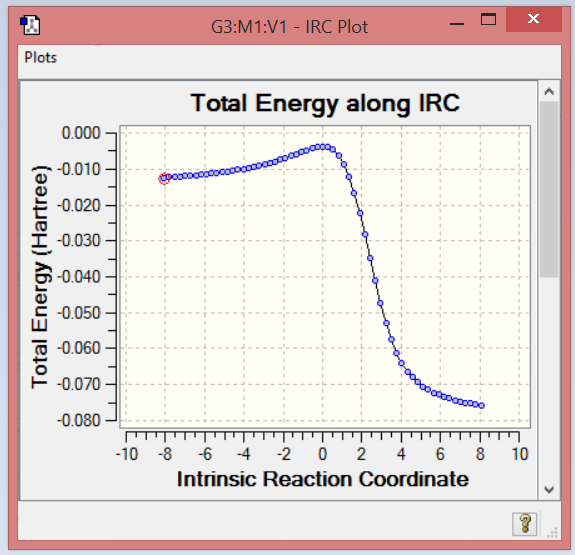
Exo diels alder IRC
File:Sh5214 3 DA exo IRC pm6.gjf
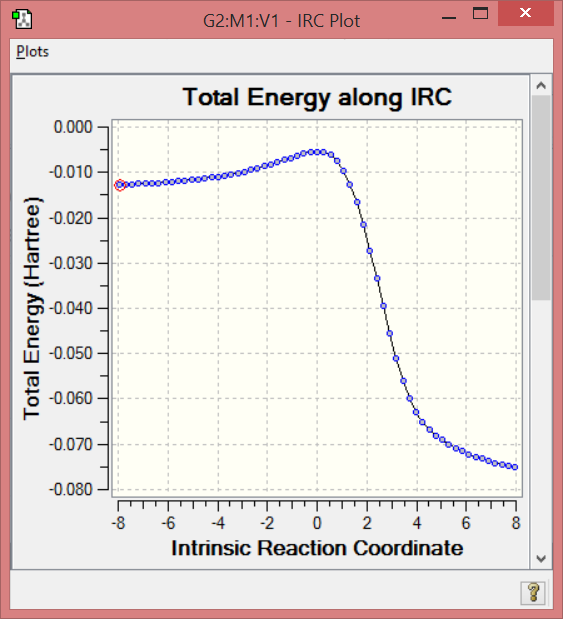
Endo diels alder IRC
File:Sh5214 3 DA endo IRC pm6.gjf
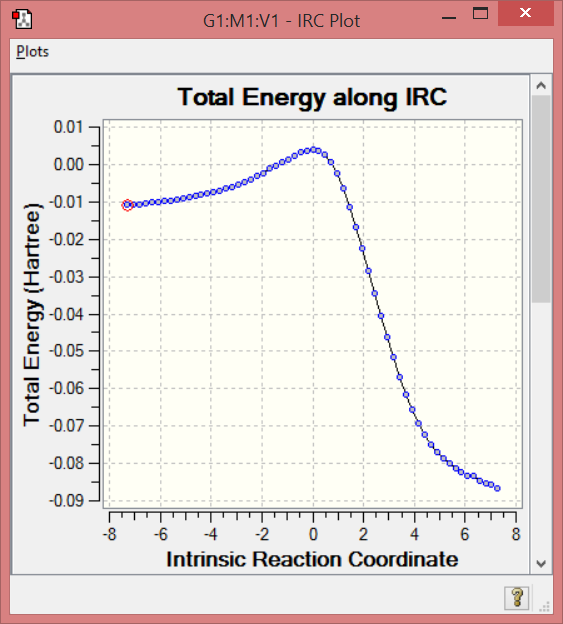
Cheletropic IRC
File:Sh5214 3 Cheletropic IRC pm6.gjf
(These IRCs are not converged. The gradient must reach 0 Tam10 (talk) 10:21, 23 March 2017 (UTC))
Thermo energies
| Sulfur dioxide (kJ/mol) | Xylylene (kJ/mol) | TS (kJ/mol) | Product (kJ/mol) | Reaction barrier (kJ/mol) | Reaction energy (kJ/mol) | |
|---|---|---|---|---|---|---|
| Exo | -311.421081 | 467.932399 | 241.748182 | 56.406246 | 85.236864 | -100.105072 |
| Endo | -311.421081 | 467.932399 | 237.762673 | 56.960227 | 81.251355 | -99.551092 |
| Cheletropic | -311.421081 | 467.932399 | 260.079424 | 0.013128 | 103.568106 | -156.498191 |
| Alternate DA exo | -311.421081 | 467.932399 | 275.819298 | 176.706665 | 119.307980 | 20.195347 |
| Alternate DA endo | -311.421081 | 467.932399 | 267.984805 | 172.259068 | 111.473487 | 15.747750 |
Endo product has the lowest reaction barrier, therefore it is the kinetic product, and the cheletropic product has the most negative reaction energy, therefore it is the thermodynamic product. It can also be seen that diels-alder reaction at the alternative diene site is unfavourable due to the fact that both the endo and exo products have a higher reaction barrier and higher reaction energy than the other 3 reactions.
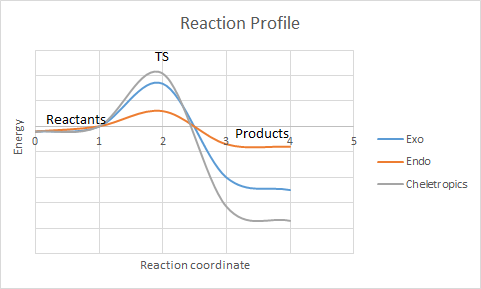
Reaction Profile
This diagram above doesn't seem to match the data you have... Endo and exo scaling don't match. It's much easier to draw straight lines between the stationary points Tam10 (talk) 10:21, 23 March 2017 (UTC))
The bonding of the 6 membered ring in xylylene goes from a diene to an 6 membered aromatic ring.
(You haven't included any of the TS geometries! Tam10 (talk) 10:21, 23 March 2017 (UTC))