Rep:Mod:seaunicorn13
Part 1: Cope Rearrangement
Optimising the reactants and products
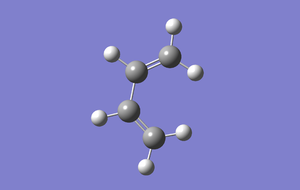
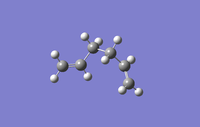
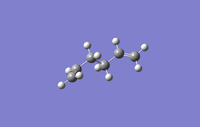
The following conformers of 1,5-hexadiene were drawn and optimised in GaussView, the given names correspond to those given in appendix 1.
| Conformer | Gauche1 | Gauche3 | Anti1 |
Anti2 |
|---|---|---|---|---|
| Structure | 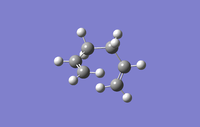 |
 |
 |

|
| Point Group | C1 | C1 | C2 | Ci |
| Energy/Hartree | -231.688 | -231.693 | -231.693 | -231.693 |
| Relative energy/kJmol-1 | 13.0 | 0 | 0.2 | 0.3 |
| File link | File:Vm1110 hexadiene gauche 1.log | File:Vm1110 hexadiene gauche 3.log | File:Vm1110 hexadiene anti 1.log | File:Vm1110 hexadiene anti 2.log |
| File Summary | File Type: .log Calculation Type: FOPT |
File Type: .log Calculation Type: FOPT |
File Type: .log Calculation Type: FOPT |
File Type: .log Calculation Type: FOPT |
The energies obtained correspond to those given in appendix 1.
Anti2 optimisation
The anti2 conformer was optimised to a higher level of theory.
Optimisation file
File:Vm1110 anti2 opt 631.log
File:Vm1110 anti2 opt 631.chk
File Type: .log
Calculation Type: FOPT
Calculation Method: RB3LYP
Basis Set: 6-31G(d)
Charge: 0
Spin: Singlet
E(RB3LYP): -234.61171020 au
RMS Gradient Norm: 0.00001448 au
Dipole Moment: 0 Debye
Point Group: Ci
Job cpu time: 45.0 Seconds
Item table
Item Value Threshold Converged?
Maximum Force 0.000021 0.000450 YES
RMS Force 0.000008 0.000300 YES
Maximum Displacement 0.000747 0.001800 YES
RMS Displacement 0.000252 0.001200 YES
Predicted change in Energy=-2.577712D-08
Optimization completed.
-- Stationary point found.
Energy and structure comparison
| HF 3-21G | DFT 6-31G* | |
|---|---|---|
| Point group | Ci | Ci |
| Energy/HF | -231.693 | -234.612 |
| Relative Energy/kJmol-1 | 7664.3 | 0 |
The energy from the B3LYP/6-31G* calculation is much lower than that from the HF/3-21G, however the structure is the same and retains the same symmetry. This shows that both levels of theory give the correct optimised structure but the higher level of theory gives a lower energy which is closer to the actual energy of the molecule in its optimised state.
Optimising the 'chair' and 'boat' transition structures
Chair TS
Allyl fragment optimisation
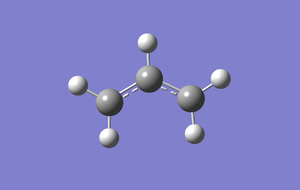
File:Vm1110 allyl opt.log
File:Vm1110 allyl opt.chk
File Type: .log
Calculation Type: FOPT
Calculation Method: UHF
Basis Set: 3-21G
Charge: 0
Spin: Singlet
E(RB3LYP): -115.82303985 au
RMS Gradient Norm: 0.00008192 au
Dipole Moment: 0.0294 Debye
Point Group: C2
Job cpu time: 5.0
Item table
Item Value Threshold Converged?
Maximum Force 0.000139 0.000450 YES
RMS Force 0.000057 0.000300 YES
Maximum Displacement 0.001133 0.001800 YES
RMS Displacement 0.000388 0.001200 YES
Predicted change in Energy=-2.083720D-07
Optimization completed.
-- Stationary point found.
Chair TS opt+freq
Two fragments were arranged such that the central C atoms on each fragment were point outwards, away from one another, forming the chair transition state. The transition state was found and optimised by using the opt+freq job type, the Hartree Frock method and the 3-21G basis set.
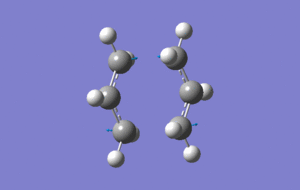
(Click the enlarge and see vibration)
File:Vm1110 chair opt.log
File:Vm1110 chair opt.chk
File Type: .log
Calculation Type: FREQ
Calculation Method: RHF
Basis Set: 3-21G
Charge: 0
Spin: Singlet
E(RB3LYP): -231.61932241 au
RMS Gradient Norm: 0.00008192 au
Dipole Moment: 0.0006 Debye
Point Group: C1
Job cpu time: 19.0
Item table
Item Value Threshold Converged?
Maximum Force 0.000051 0.000450 YES
RMS Force 0.000009 0.000300 YES
Maximum Displacement 0.001795 0.001800 YES
RMS Displacement 0.000458 0.001200 YES
Predicted change in Energy=-6.233796D-08
Optimization completed.
-- Stationary point found.
The imaginary frequency shown in the above image for the transition state occurs at -818cm-1 and shows the bond formation in the transition state.
Boat TS
The boat transition state was optimised by using the optimised anti2 conformer of 1,5-hexadiene and performing an opt+freq calculation using QST2 method. The first attempt with the optimised conformer with no alternations failed so the dihedral angle of the central C-C bond was rotated by 180° so that the molecules more closely resembled the boat transition state and could be more easily optimised.
Failed Boast TS optimisation
File:Vm1110 boat opt failed.log
File:Vm1110 boat opt failed.chk
Successful Boast TS optimisation
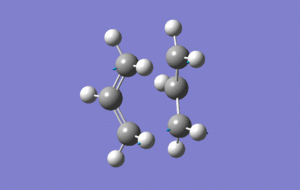
(Click the enlarge and see vibration)
File:Vm1110 boat opt.log
File:Vm1110 boat opt.chk
File Type: .log
Calculation Type: FREQ
Calculation Method: RHF
Basis Set: 3-21G
Charge: 0
Spin: Singlet
E(RB3LYP): -231.60280239 au
RMS Gradient Norm: 0.00003250 au
Dipole Moment: 0.1584 Debye
Point Group: CS
Job cpu time: 29.0
The imaginary frequency shown in the above image for the transition state occurs at -840cm-1 and shows the bond formation in the transition state.
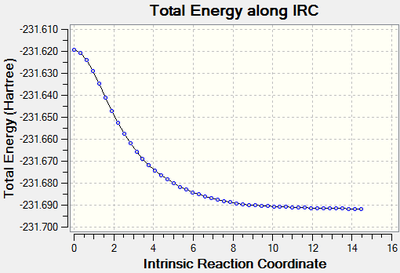
IRC
An IRC calculation was performed on the optimised chair transition structure to confirm that the transition state was a maximum energy.
IRC
File:Vm1110 chair irc.log
(checkpoint file too large to upload to wiki)
File Type: .log
Calculation Type: IRC
Calculation Method:
Basis Set:
Charge: 0
Spin: Singlet
E(RB3LYP): -231.61932241 au
RMS Gradient Norm: 0.00001799 au
Dipole Moment: 0.0006 Debye
Point Group: C1
Job cpu time: 16 minutes 38.0 seconds
The graph above shows that the transition state found does indeed occupy the highest energy, hence it is the transition state. The IRC was calculated only in the forward direction because for this molecule it is symmetric.
Activation Energies
Summary of energies (in au)
| HF 3-21G | B3LYP 6-31G* | |
|---|---|---|
| Electronic energy | Electronic energy | |
| Chair TS | -231.61932241 | -234.5575872 |
| Boat TS | -231.60280239 | -234.5436851 |
| Anti2 | -231.69253516 | -234.61171020 |
Summary of activation energies (in kJmol-1)
| HF 32-1G at 0K |
B3LYP 6-31G* at 0K |
Expt | |
|---|---|---|---|
| Chair | 192.6 | 142.1 | 140.2 ±2 |
| Boat | 235.9 | 178.6 | 187.0 ±8 |
As can be seen, the results from the higher level of theory are much closer to experimental values.
Part 2: Diels Alder
Cis butadiene optimisation
Optimisation file
File:Vm1110 butadiene opt.log
File:Vm1110 butadiene opt.chk
File Type: .log
Calculation Type: FOPT
Calculation Method: RAM1
Basis Set: ZDO
Charge: 0
Spin: Singlet
E(RB3LYP): 0.04879719 au
RMS Gradient Norm: 0.00001742 au
Dipole Moment: 0.0414 Debye
Point Group: C2V
Job cpu time: 2.0 seconds
Item table
Item Value Threshold Converged?
Maximum Force 0.000030 0.000450 YES
RMS Force 0.000011 0.000300 YES
Maximum Displacement 0.000442 0.001800 YES
RMS Displacement 0.000164 0.001200 YES
Predicted change in Energy=-8.983482D-09
Optimization completed.
-- Stationary point found.
Molecular Orbitals
| HOMO | LUMO | |
|---|---|---|
| Orbital | 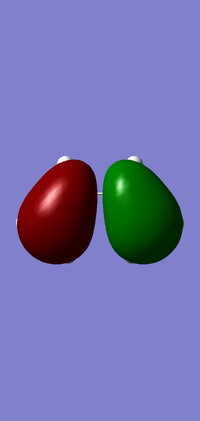 |
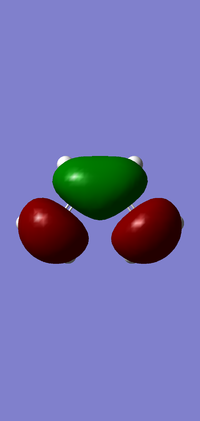
|
| Symmetry | Asymmetric | Symmetric |
Prototype reaction transition state
The transition state was investigated for the following reaction:
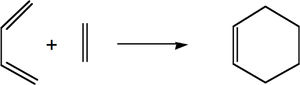
The correct geometry was found by drawing and cleaning up the bicyclo structure and removing one of the added ethylene fragments. The transition state was optimised and characterised using the opt+freq job type. The method used was semi-empirical AM1, as for the optimisation above.
Optimisation/frequency file

File:Vm1110 prototype ts.log
File:Vm1110 prototype ts.chk
File Type: .log
Calculation Type: FREQ
Calculation Method: RAM1
Basis Set: ZDO
Charge: 0
Spin: Singlet
E(RB3LYP): 0.11165464 au
RMS Gradient Norm: 0.00000030 au
Dipole Moment: 0.5605 Debye
Point Group: C1
Job cpu time: 3.0 seconds
Item table
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000028 0.001800 YES
RMS Displacement 0.000006 0.001200 YES
Predicted change in Energy=-9.870468D-12
Optimization completed.
-- Stationary point found.
HOMO
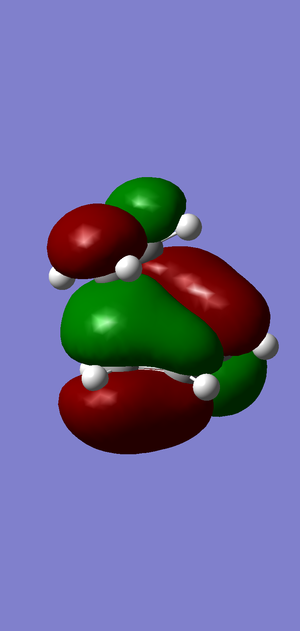
Asymmetric. Formed from the HOMO of the cis butadiene (AS, as seen above)) and the LUMO of the -CH2-CH2- fragment.
LUMO
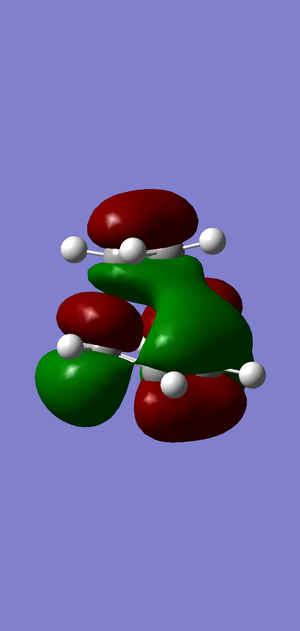
Symmetric. Formed from the LUMO of the cis butadiene (S, as seen above)) and the HOMO of the -CH2-CH2- fragment.
Discussion
The optimisation converged and the frequency file gave a single imaginary vibration at -956cm-1 corresponding to the bond formation. The frequency also gave thermochemical information about the transition state, shown below.
Sum of electronic and zero-point Energies= 0.253276 Sum of electronic and thermal Energies= 0.259453 Sum of electronic and thermal Enthalpies= 0.260397 Sum of electronic and thermal Free Energies= 0.224015
Diels Alder reaction with maleic anhydride
Maleic anhydride reacts with cyclohexa-1,3-diene in a diels alder mechanism to give an endo and an exo product. The reaction is kinetically controlled. Below the energies of the transition states for both the endo and the exo forms are compared.
Endo transition state

Optimisation
File:Vm1110 endo ts.log
(Checkpoint file too large to put in wiki)
File Type: .log
Calculation Type: FREQ
Calculation Method: RAM1
Basis Set: ZDO
Charge: 0
Spin: Singlet
E(RB3LYP): -0.05150480 au
RMS Gradient Norm: 0.00000027 au
Dipole Moment: 0.5605 Debye
Point Group: C1
Job cpu time: 5.0 seconds
Item table
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000023 0.001800 YES
RMS Displacement 0.000003 0.001200 YES
Predicted change in Energy=-5.113571D-12
Optimization completed.
-- Stationary point found.
Exo transition state
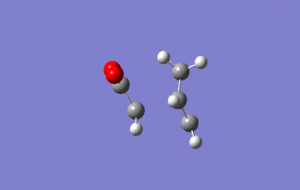
Optimisation
File:Vm1110 exo ts.log
(Checkpoint file too large to put in wiki)
File Type: .log
Calculation Type: FREQ
Calculation Method: RAM1
Basis Set: ZDO
Charge: 0
Spin: Singlet
E(RB3LYP):-0.05041968 au
RMS Gradient Norm: 0.00004220 au
Dipole Moment: 5.5647 Debye
Point Group: C1
Job cpu time: 11.0 seconds
Item table
Item Value Threshold Converged?
Maximum Force 0.000122 0.000450 YES
RMS Force 0.000017 0.000300 YES
Maximum Displacement 0.001782 0.001800 YES
RMS Displacement 0.000188 0.001200 YES
Predicted change in Energy=-1.720725D-07
Optimization completed.
-- Stationary point found.
Discussion
| Endo | Exo | |||
|---|---|---|---|---|
| MOs | 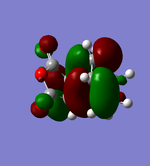 HOMO |
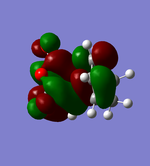 LUMO |
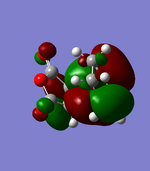 HOMO |
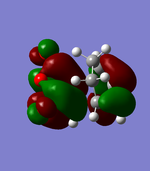 LUMO |
As can be seen in the above table, the central oxygen on the maleic anhydride contributes less to the overall bonding and antibonding of the LUMO and HOMO for the exo product than the endo product. This is due to oxygen being highly electronegative.
| Endo | Exo | |
|---|---|---|
| partially formed σ C-C bond | 2.162 | 2.170 |
| C-C through space distances | 2.892 | 2.945 |
| C-C single bond in cyclohexa-1,3-diene | 1.393 | 1.394 |
| C-C single bond in maleic anhydride | 1.489 | 1.488 |
The carbon Van der Waal radius of carbon is 1.7, compared to this the C-C distance through space is much shorter than the sum of two carbon Van der Waal radii. This means that there are forces holding the carbons closer together. This is due to secondary orbital overlap effect. This is more pronounced for the endo transition state which explains why it is lower in energy.
| Endo | Exo | |
|---|---|---|
| Energy/HF | -0.052 | -0.050 |
| Relative Energy/kJmol-1 | 0 | 2.849 |
These relative energies show that the endo form is lower in energy and therefore more stabilised.
Ending comments
All of these calculations neglected solvent effects on the transition states and reactions. Solvents significantly effect the stability of transition states and hence change which product is more likely in a reaction.