Rep:Mod:sb1106
Shahania Begum
The application of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
Allinger MM2, the molecular mechanics model is the parametric technique which was used to optimise the molecular geometry of the following compounds which were investigated in order to minimise the energy of the compound. After which the bond length, angle strain, steric effects and van der Waals contributions were analysed.
The Hydrogenation of Cyclopentadiene Dimer
When cyclopentadiene dimerises the endo dimer 2 rather than the exo dimer 1 is specifically produced. Hydrogenation of this dimer proceeds to give initially one of the dihydro derivatives 3 or 4.

MM2 was applied to all four species and their energies and geometries were calculated;
| Molecule | Endo 1 | Exo 2 | 3 | 4 |
| Stretch | 1.2882 | 1.2611 | 1.2729 | 1.1034 |
| Bend | 20.5789 | 20.8214 | 19.8834 | 14.5151 |
| Stretch-Bend | -0.8389 | -0.8408 | -0.8339 | -0.5464 |
| Torsion | 7.6732 | 9.5211 | 10.7965 | 12.5123 |
| Non-1,4 VDW | -1.4199 | -1.5452 | -1.2325 | -1.0470 |
| 1,4 VDW | 4.2280 | 4.3363 | 5.6403 | 4.4985 |
| Dipole/Dipole | 0.3781 | 0.4488 | 0.1621 | 0.1408 |
| Total Energy kcal/mol | 31.8878 | 34.0026 | 35.6887 | 31.1767 |
From comparing the final energies of compounds 1 and 2, it can be seen that the endo conformer 1, is lower in energy and could possibly be the thermodynamically more stable product and therefore might have been expected to be the major product however this is not the case.
It could be assumed that the major exo conformer 2, is the product of kinetic control. This may be due to the endo intermediate which was formed being lower in energy than the exo intermediate. Looking at the relative energies of the hydrogenated exo dimers, dimer 4 is slightly lower in energy than exo dimer 3.Dimer 3 may be higher in energy due to additional ring strain caused by the double bond in the bicyclic system. From comparing the stretches, bends, torsions , VDW and dipole-dipole interactions where the hydrogentated compounds differ is in the bending mode otherwise these above modes and interactions are very similar. The exo dimer 4 appears to be much more flexible, with a bending strain 27% smaller than that of dimer 3.
Both these factors could be reasons to support that hydrogenation is a thermodynamic process leading to exo dimer 4 being the major product.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
In this first example, the optically active prolinol 5 reacts with methyl magnesium iodide which can alkylate the pyridine ring at either the 4 position or at the 2 position, however the major product is 6a, which is formed under chelate control whereas 6b only forms under random addition.

The above reaction seems to proceed via the following mechanism, 6a is the major product as the reaction proceeds via a six membered transition state where there is intramolecular chelation control via the coordination of the Grignard reagent to the amide oxygen whereby the Methyl group preferentially attacks at the 4 position closest to the carbonyl group.

The method of MM2 was applied to optimise the geometry of the structures and the following data was obtained;
| Molecule |
|
| ||||||
| Stretch | 0.8776 | 0.9236 | ||||||
| Bend | 5.7820 | 8.3562 | ||||||
| Stretch-Bend | 0.0878 | 0.0325 | ||||||
| Torsion | 9.9152 | 9.9863 | ||||||
| Non-1,4 VDW | -3.0835 | -3.0223 | ||||||
| 1,4 VDW | 11.6906 | 12.5069 | ||||||
| Charge/Dipole | 3.4546 | --- | ||||||
| Dipole/Dipole | -3.7399 | -4.0532 | ||||||
| Total Energy kcal/mol | 24.9844 | 26.5903 |
The conformational minima was achieved by orientating the carbonyl group of the amide above the plane of the pyridine ring anti to the H-atom at the chiral centre. The carbonyl group is conformationally locked and cannot be taken below the plane. The carbonyl group is no co planar with the aromatic ring system, hence the dihedral angle in the molecule is 24.3247°. If the system was planar the dihedral angle would be 0°.
In the second example, the pyridinium ring of 7 is reacted with aniline, the NHPhenyl group nucleophilically added to give product 8.

MM2 was applied and the following data was obtained;
| Molecule |
|
| ||||||
| Stretch | 1.5267 | 1.8970 | ||||||
| Bend | 6.7001 | 13.5622 | ||||||
| Stretch-Bend | 0.3347 | 0.6235 | ||||||
| Torsion | -5.9842 | -12.0112 | ||||||
| Non-1,4 VDW | -2.5269 | -2.4523 | ||||||
| 1,4 VDW | 17.7017 | 20.4581 | ||||||
| Charge/Dipole | 2.3815 | --- | ||||||
| Dipole/Dipole | -4.8266 | -4.7899 | ||||||
| Total Energy kcal/mol | 15.3069 | 17.9200 |
There is very little flexibility in 8, due to the seven membered ring. The chiral masked amides, with a C3 axis through the C=O undegergoes a 1,4 reduction. The origin of this stereocontrol in the formation of 8 is a consequence of the NHphenyl nucleophile attacking from the opposite face in which the carbonyl is pointing due to sterics as well as avoiding unfavourable repulsion between the lone pair on the amide carbonyl and the attacking nucleophile. In this example the amide carbonyl group in compound 7 point down from the plane of the aromatic ring system and the observed dihedral angle again is not zero but 41.7805°.
Overall the technique of MM2 is a good interpolative technique however as the above exercises have demonstrated; MM2 is not very useful in modelling reactions which are governed by ‘kinetic control’ as MM2 cannot provide the necessary information such as transition state or intermediate structures. Perhaps if factors such as energies of transition states and activation barriers could be calculated a better evaluation of determining whether the reactions are under kinetic or thermodynamic control could be obtained.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Taxol is an important drug in the treatment of ovarian cancers, it is initially synthesised with the carbonyl group pointing either up or down.
Atropisomerism is observed, on standing the compound apparently isomerises to the alternative carbonyl isomer. Below 10 and 11 are key intermediates in the total synthesis of taxol.
MM2 was applied to both intermediates in order to identify the more stable isomer.
Clearly the stereochemistry of carbonyl addition depends on which isomer is the most stable. Intermediate 10 has the methylene bridgehead cis to carbonyl group. Whereas intermediate 11 has the methylene bridgehead trans to the carbonyl group. The energy of intermediate 11 was lowered from about 90 to 45 Kcal/mol by altering the position of the carbonyl group, as well as removing the hydrogen atoms individually and then rectifying the compound in order to try and minimise the energy. It doesn’t say where the strain is located, however isomer 10 is still slightly lower in energy, and however this very small difference in energy is the reason why they can interconvert easily.
Intermediate 11 has a greater torsional strain; however more sophisticated software would be needed to identify the exact position of this additional strain. Intermediate 10 is a hyperstable olefin which is more stable next to an unreactive bridgehead as well as having a larger ring, resulting in less strain compared to 11.
DOI; look up hyperstable alkenes.
How one might induce room temperature hydrolysis of a peptide
Conformational analysis can be used to rationalize kinetic behaviour. Some enzymes can achieve hydrolysis of a peptide in less than 1 second. The two cis and trans isomers 13 and 14 shown below differ only in their stereochemistry, yet the cis isomer reacts much faster than the trans.
For peptide 13, with the N-substituents orientated axially or equatorially with respect to the decalin ring have been modelled below.

The technique of MM2 was applied to the two possible cis isomers and the following energies and data were calculated;
| Molecule |
|
| ||||||
| Stretch | 1.4094 | 1.7017 | ||||||
| Bend | 7.6831 | 5.1778 | ||||||
| Stretch-Bend | 0.6518 | 0.5308 | ||||||
| Torsion | 11.6847 | 9.3480 | ||||||
| Non-1,4 VDW | -8.2465 | -7.3944 | ||||||
| 1,4 VDW | 10.0577 | 10.0362 | ||||||
| Dipole/Dipole | -4.6461 | -6.2657 | ||||||
| Total Energy kcal/mol | 19.7945 | 13.1344 |
For the Trans isomer where the N-substituent was either equatorial or axial was modelled using MM2;

The technique of MM2 was applied to the two possible trans isomers and the following energies and data were calculated;
| Molecule |
|
| ||||||
| Stretch | 1.5550 | 1.4617 | ||||||
| Bend | 5.3437 | 3.8118 | ||||||
| Stretch-Bend | 0.5579 | 0.5034 | ||||||
| Torsion | 9.4766 | 7.7140 | ||||||
| Non-1,4 VDW | -4.9948 | -7.1723 | ||||||
| 1,4 VDW | 9.4326 | 9.9281 | ||||||
| Dipole/Dipole | -4.5814 | -6.5699 | ||||||
| Total Energy kcal/mol | 16.7896 | 9.6767 |
The trans isomer is more stable than the cis isomer and in both cases the equatorial conformer for the N- substituent is favoured and are lower in energy. Literature confirms this.
INSERT DOI
The cis isomer undergoes pseudo A1,3 release of strain, between the pyrrolidine ring and adjacent methyl group. Intramolecular reactions react rapidly in order to relieve strain. The mechanism for this reaction involves the hydroxyl group acting as nucleophile which is activated by the amino group on the N-substituent.
A successful reaction requires the hydroxyl group to be close enough to the peptide bond as well as at the right angle of attack of 108° so that the oxygen lone pair has the best overlap with the π* C=O orbital. For the trans isomer the N-substituent has to be axial to be in position for the right orientation for attack however it costs energy for the conformer to switch from equatorial (the more stable conformer) to axial therefore it is much slower in reacting.
Modelling Using Semi-empirical Molecular Orbital Theory

MM2 , and Hartreefock MO methods were both applied to the above molecule (12) 9- ChIoro-1,4,5,8- tetrahydro-4a,8a –methanonaphthaIene, this provided an approximate representation of the valence-electron molecular wavefunction, and in particular of the HOMO. The geometry was then calculated using a more accurate Density-functional B3LYP/6-31G(d) approach which generated the following molecular orbitals;
| Homo | Homo-1 | Homo-2 |
 |
 |
 |
| Lumo | Lumo+1 | Lumo+2 |
 |
 |
 |
The following two molecules have been compared;
 |
 |
Compound 12 which contains a double bond anti to the Cl-C bond and a hydrogenated version where this anti (or exo) double bond is replaced by a C-C single bond. The geometries of both compounds were optimized, and the vibrational frequencies of each were calculated. The following IR spectra were generated for each molecule;
IR Spectra for compound 12 http://hdl.handle.net/10042/to-1733
IR Spectra for the monohydrogenated derivative of compound 12 http://hdl.handle.net/10042/to-1734
Analysing IR spectra Compound 12 contains a Cl-C stretching frequency at 772.634 cm-1, with the highest intensity compared to the other Cl-C stretches with an IR intensity of 25.2418.The two C=C stretches are at 1740.76 cm-1 with an IR intensity of 4.1445 and at 1760.91 cm-1 with an IR intensity of 3.9036. Monohydrogenated derivative of compound 12 contains a Cl-C stretching frequency at 769.964 cm-1, with the highest intensity compared to the other Cl-C stretches with an IR intensity of 19.4253. There is one C=C stretches at 1740.61 cm-1 with an IR intensity of 4.1801.
The C-Cl stretch is at a slightly lower frequency for the monohydrogenated compound compared to compound 12 therefore it absorbs less strongly than for compound 12.
Structure based Mini project using DFT-based Molecular orbital methods
For this organic structure based mini project, the following reaction has been chosen from primary literature as two possible products could be produced; The reaction below involves the formation of bridged bicyclic dienones via silyl directed Nazrov cyclisation processes which are highly diastereoselective. It was investigated the reasons for which this process prefers an exo-disposed cyclopentanone over the endo configuration and a case where this preference may be reversed.
This reaction is stereospecific in forming the exo diastereomer majorly with a small amount of the silyl containing product.
Literature DOI Nazrov DOI

The endo diastereomer below is not formed, it has been investigated as to the reason why the exo conformer is preferentially formed.

The two diastereomers have rigid structures due to the double bond in the seven membered ring and the methylene bridgehead which disallows any ring flipping between the fused 5 and seven membered rings into chair or twist boat like structures. This is very helpful in obtaining the optimized and lowest energy conformation. Several computational methods have been applied and steric and electronic effects have been taken into account in trying to rationalise why the exo product is favoured over the endo product and whether the reaction is under kinetic or thermodynamic control using several computational techniques. MM2 was first applied to both diastereomers in order to geometry optimise the structures of both diastereomers after which, Hartree Fock method were also applied and then DFT method was applied using SCAN, The following energies and geometry data were obtained After MM2

After applying MM2 it appears as though the exo isomer is much higher in energy and the least thermodynamically stable of the two diastereomers. However after running HF and DFT optimisation techniques the following energies were obtained;
Exo 21.8820 kcal/mol
Endo 23.3662 kcal/mol
The change in pushing the cyclopentanone ring almost perpendicular to the seven membered ring with the exo protons facing straight in a cis orientation with the bridgehead allows the protons to be closer in orientation to the bridgehead methyl groups, must be a favourable stabilising interaction as this optimized geometry resultant from DFT optimisation demonstrates how powerful this technique is compared to MM2, taking electronic and steric contributions into account, the energy of the exo product was reduced from 81 Kcal/mol to 21.88kcal/mol, which is only just slightly lower than that for the endo product.
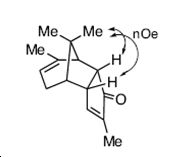
As the computational techniques applied have shown the exo isomer showed clear nOe interactions consistent only with the indicated stereochemistry this stabilising interaction is not present in the endo distereomer and this may be why the exo conformer is slightly lower in energy compared to the endo isomer.
In order to differentiate spectroscopically between the diastereoisomers, ideally 1HNMR spectroscopy would be used and the key differences which would be used to identify the two samples would be certain trends such as the bridgehead protons of the endo isomer would be expected to be 0.5-1.0ppm further downfield than those of the exo isomers, this would presumably be due to anisotropy from the C=C bond in the cyclopentanone ring. Also X-ray crystallography would be useful in determining the exact structure and coordination of atoms.
As such computational techniques cannot compute proton NMR shifts with a great deal of accuracy the 13C spectra for the isomers using the GIAO method has been predicted and compared to that of literature in order to see whether they match and or support the structural assignments of literature.
Comparing EXO AND ENDO 13C NMR
| Exo | Endo | ||||||
 |
 | ||||||
|
|
From comparing the 13C NMR chemical shifts of the exo compound to that of literature it can be seen that this technique produces results with a high degree of accuracy very close to experimental values from literature with an error of +/- 3ppm. There is no literature for the endo diastereomer, however due to the high degree of accuracy of the calculations the chemical shifts for endo and exo can be compared. Where the main differences between the chemical shifts for the exo and endo conformers are for carbons 1 and 3 for the endo isomer, these chemical shifts appear more downfield than for the exo isomer. This could be due to the varying geometry, for the exo isomer the bond angle between atoms 1, 3 and 4 is 112° whereas that same angle is slightly smaller at 108° for the endo isomer this means that the geometry is for the endo isomer around carbon 3 is similar to being tetrahedral thereby being closer in proximity to neighbouring carbons resulting in higher chemical shifts. There were no spin-orbit coupling errors to consider as there were no heavy elements attached to the carbons.

The optical rotation was calculated for both isomers, the following data was obtained; Endo optical rotation Optical Rotation Beta= -0.4316 au.
Molar Mass = 216.3224 grams/mole, [Alpha]D = -77.12°.
Exo optical rotation Optical Rotation Beta= 1.0306 au.
Molar Mass = 216.3224 grams/mole, [Alpha]D = 184.14°.
The values are very different and considered very inaccurate. The optical rotation of the compound was not stated in literature however the sign/ (R) or (S) configuration was stated with the exo isomer being (R) therefore the endo conformer is (S). The optical rotation may not be useful in providing an accurate angle however it is useful in providing the accurate sign/orientation with the endo isomer being (-) and exo isomer being (+). The IR spectra of the two diastereomers were computed and their vibrational frequencies analysed and compared. This is very useful as a paraffin mull does not have to be used and all of the vibrational frequencies can be linked back to the particular mode which causes the absorption. Literature only gives two absorbance frequencies; (C-H) 2917 and (C=C)1699 cm-1 These shall be compared to the ones computed for the endo and exo isomer, below are the IR spectra of the exo and endo products;
Endo

Exo

The stretching frequencies of with the greatest IR intensities were compared; visually they look almost identical however analysis of each vibrational modes brings slight variations in absorbancies to our attention.

It can be seen that the strongest absorbance is that of the (C=O) stretch which is similar for both isomers however it appears that the (C=O) stretch in the endo compound absorbs more strongly than the exo with a grater IR intensity this suggests a stronger bond. Whereas the (C=C) stretch caused by the double bond in the cyclopenatone ring absorbs more strongly for the exo compound however only just slightly. The methyl stretches are very similar. This computational technique is very useful and quite accurate in obtaining values comparable to literature. From calculating the Gibbs Free energy of both isomers this could potentially inform us of which isomer is the most thermodynamically stable isomer,
Exo ∆G -413279.99 kcal/mol
Endo ∆G -413270.20 kcal/mol
The change in gibbs free energy between the two isomers is 9.79 kcal/mol, the exo isomer in agreement with the energies computed by DFT optimisation confirm the exo isomer to be slightly higher in energy and therefore thermodynamic control may be a contributing factor in the stereospecificity of the reaction in the formation of the exo isomer.
In conclusion, there doesn’t seem to be a particular technique which can support or evidently conclude the selectivity of this particular reaction however this is the proposed mechanism;

The selectivity is due to the favourable cis geometry of the protons and the nOe interaction. Perhaps taking a look at the molecular orbitals and their overlap and interactions maybe a good area to look into if we were given more time. Overall it can be seen how computational techniques can be useful in some cases in helping to differentiate between isomers however additional techniques still need to be used.
References
- A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- Leleu, Stephane; Papamicael, Cyril; Marsais, Francis; Dupas, Georges; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928. DOI:10.1016/j.tetasy.2004.11.004
- S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- See J. G. Vinter and H. M. R. Hoffman, J. Am. Chem. Soc., 1974, 96, 5466 (DOI:10.1021/ja00824a025 DOI:10.1021/ja00824a025 ) and 95, 3051 for another nice example of atropisomerism.
Mini Project
- Stereoselective Nazarov Cyclizations of Bridged Bicyclic Dienones Robert D. Mazzola, Timothy D. White, Heidi R. Vollmer-Snarr, and F. G. West Org. Lett., 2005, 7 (13), 2799-2801 • DOI:10.1021/ol051169q
NMR endo
DOI http://hdl.handle.net/10042/to-1738
NMR exo DOI http://hdl.handle.net/10042/to-1638
IR endo DOI http://hdl.handle.net/10042/to-1741
IR exo DOI http://hdl.handle.net/10042/to-1740


