Rep:Mod:santacruz
The basic techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
The Dimerisation of Cyclopentadiene
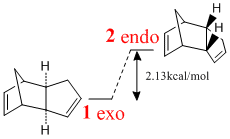
Cyclopentadiene exists as a dimer at room temperature[1] and has been shown to dimerise via a Diels-Alder type п4s+п2s cycloaddition which will give two isomers depending on the orientation of the molecules.
Using MM2 dynamics from ChemBio3D, the relative energy difference between both isomers of the product can be calculated. These calculations show that the exo form is the thermodynamic product. The endo form is 2.13kcal/mol (6.6%) more unstable. The main contributor to this instability is an increase of 1.86kcal/mol (24.3%) in the torsional energy of the molecule.
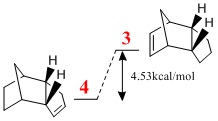
Upon hydrogenating the endo dimer, one of the double bonds is reduced preferentially over the other. MM2 calculations shows that molecule 4 is the most stable thermodynamic product. Product 3 is 4.53kcal/mol (14.5%) more unstable. The contributions to this instability are shown in the table on the right.
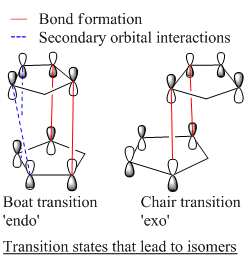
Empirical evidence however, shows that the endo configuration is the major product. This means that the reaction is kinetically controlled. Specifically, it's not the orbitals directly involved in forming the new bonds that determine the configuration. So called secondary orbital interactions first proposed by Woodward and Hoffmann [2][3][4] help the molecules into a given position as shown in the image to the left.
The red lines demonstrate the position of the bond that is to be formed. The blue dashed lines however indicate secondary orbital interactions which occur in the endo configuration and not exo. The extra stabilisation of the endo transition state means the energy barrier is lowered enough for this to be the major isomer.
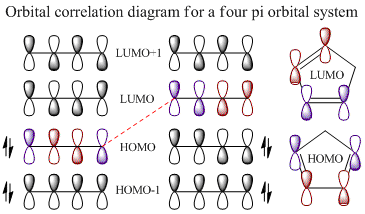
An orbital correlation diagram highlights the allowed orbital combination due to Huckel's rules[5] of a six electron cycloaddition under thermal conditions where orbitals will only interact if their symmetry match. The blue frontier orbitals (ones directly taking part in bond formation) both have the same rotational symmetry. The red orbitals don't directly take part in the reaction; instead they are free to interact via secondary orbital interactions.
The four images below are 3D visualisations of the four pi-system orbitals shown qualitatively above. They clearly display the HOMO-1 having all four pi orbitals in the same phase, the HOMO having one node, the LUMO having two nodes and the LUMO+1 having a strongly anti-bonding system. These were calculated using ChemBio3D's MOPAC/PM6 algorithm.
HOMO-1 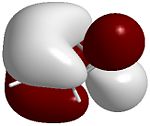 |
HOMO 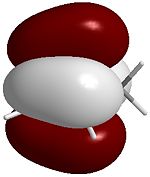 |
LUMO 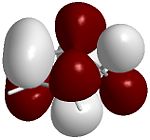 |
LUMO+1 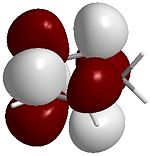 |
|---|
The Hydrogenation of Dicyclopentadiene
Dicyclopentadiene is hydrogenated using a palladium catalyst [6] into the dihydro form before forcing it into the tetrahydro form. The relative stabilities of dihydro isomers 3 and 4 can give us an insight into which will double bond will be saturated first.
To do so, MM2 calculations of both hydrogenated isomers are used to give data on the contribution to their relative energies as shown in the table below.
From the MM2 calculation, it can be seen that 4 is 4.53kcal/mol, or 14.5% more stable than 3. The major contribution to this decrease in stability going from 4 to 3 is a 5.34kcal/mol increase due to "Stretch-Bend" which means that the bridgehead methylene increases the strain on the double bond more than it does a single bond.
The above statement is reinforced by using the MM2 optimised structure to determine the C=C-C angle which is 107.7o for 3 compared to 112.4o for 4. They are both more strained than the 120o found on a regular sp2 centre.
The greater thermodynamic stability of 4 means that the first alkene to be hydrogenated is the syn-alkene (endo with respect to the methylene bridge).
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
Including the MeMgI in the MM2 calculations results in an error. This is probably due to the parameters used in the MM2 algorithm doesn't take into account metal atoms[7]. An improved mathematical model with force fields that take into account metals could be used to calculate the effect of having a Magnesium atom interacting with the molecule.
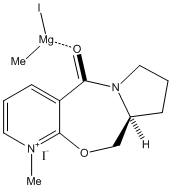
The reaction of 5 with MeMgI proceeds by a a metal-oxygen interaction that keeps the attacking methyl group on the side of the carbonyl group[8].
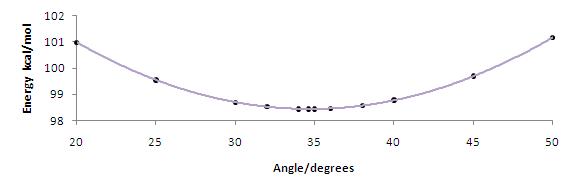
The following images are dihedral plots that calculate a conformational minimum, as calculated using MMFF94, when the carbonyl is 4.1o from the plane of the aromatic system for 5 and 34.6o from the plane of the aromatic system for 7. The corresponding absolute energies are 57.4kcal/mol and 98.4kcal/mol respectively.
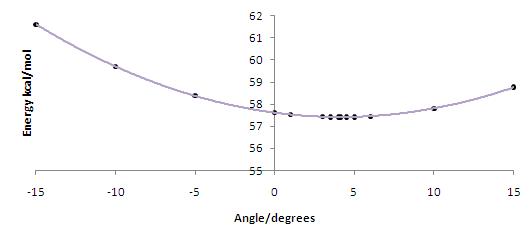
The slight angle of 4.1o is sufficient so as to allow the metal to chelate with the carbonyl, producing an enantiomeric excess of the product. The reaction proceeds via a six-membered transition state as illustrated below:
Using the same approach as above, the geometry of reactant 7 was optimised using MMFF94 and the energies of several dihedral angles were plotted as seen in the image below:
As the carbonyl angle is much sharper from the plane of the aromatic system, it can interact with hydrogen bonding to the amine producing a result similar to that of 5 and MeMgi:
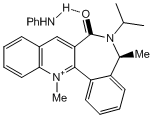
In order to improve these calculations, a more advanced algorithm could be implemented to take into account hydrogen metal atoms. As the carbonyl angle varied greatly with the overall energy, a good geometric model is required. This can be achieved using the SCAN and a larger basis set.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
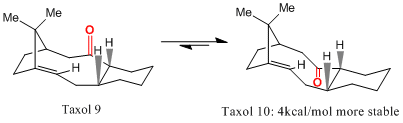
Taxol contains a 'hyperstable' bridgehead alkene[9]. Hyperstable olefins are ones that have a negative OS (olefin strain) value; the unsaturated version of the molecule has less strain placed upon it than the parent alkane. On bridgehead alkenes, this effect coupled with the steric bulk around the double bond gives it a high resistance to hydrogenation. This is a useful feature if attack to the bond is undesired.
Taxol displays an atropisomer, the carbonyl can either point syn to the hydrogen atoms shown with wedged bonds or anti to them. This configurational isomer interconverts at room temperature from 9 to 10.
Whilst optimising taxol, it was noted that changing the conformation of the six membered ring from a twist-boat to a chair afforded an energy drop of 4~5kcal/mol. The transition occurs slowly as the barrier to conversion is around 8 times larger than the thermal energy accesible to it due to room temperature (0.59kcal/mol).
The table below shows the MM2 optimised energy contributions for both isomers.
| Contribution | Energy of 9 kcal/mol | Energy of 10 kcal/mol | Difference kcal/mol | % increase |
|---|---|---|---|---|
| Stretch: | 2.68 | 2.57 | 0.11 | 4.18 |
| Bend: | 26.82 | 10.70 | 5.12 | 32.34 |
| Stretch-Bend: | 0.40 | 0.32 | 0.08 | 20.3 |
| Torsion: | 18.20 | 19.74 | -1.54 | -8.45 |
| Non-1,4 VDW: | -1.02 | -1.40 | 0.39 | -37.81 |
| 1,4 VDW: | 12.65 | 12.54 | 0.11 | 0.87 |
| Dipole/Dipole: | 0.15 | -0.18 | 0.33 | 220.17 |
| Total | 48.89 | 44.29 | 4.60 | 9.41 |
As shown, 10 is 4.60kcal/mol (9.41%) more stable than 9. This can be accounted for by the largest contribution to stability: "Bend energy". The increase in bend energy is because 9 has a C-CO-C angle of 126.2o as opposed to the unstrained 120.0o angle displayed by the carbonyl in 10.
Using the MMFF94 method, the structures undergo a negligible change in configuration and 10 is calculated to be 10.03kcal/mol more stable than 9.
Modelling Using Semi-empirical Molecular Orbital Theory
Part 1
12 using MM2 is 17.9040 kcal/mol MOPAC: Heat of Formation = 19.74056 Kcal/Mol By using a quick MM2 calculation followed by a MOPAC/PM6 calculation, an approximation of the molecular orbitals of 12 can be deduced. The following images are graphical representations of these orbitals:
Skeletal structure  |
HOMO-1 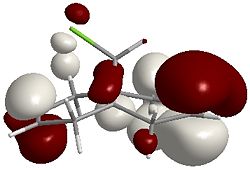 |
HOMO 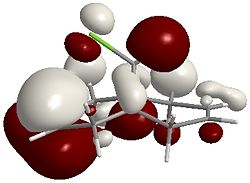 |
|---|---|---|
LUMO  |
LUMO+1 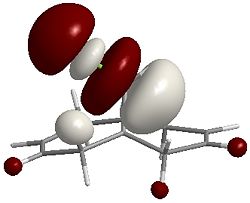 |
LUMO+2 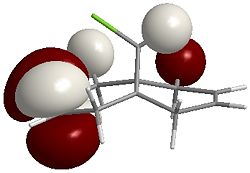 |
It can be seen that the majority of the electron density of the HOMO lies on the side (endo) of the Chlorine (indicated by the green cylinder on the top LHS of the images), whereas the LUMO shows orbitals that are weighted towards the opposite side (exo). As a reaction with dichlorocarbene is electrophilic, the electron-rich olefin dictates the selectivity and so explains why carbene attacks the endo alkene.
Theoretically, if the HOMO-1 was of higher energy than the HOMO, we'd expect electrophilic attack at the exo alkene as the electron density is clustered on the opposite side to the Chlorine atom.
Part 2
Vibrational frequencies depend on the strengths of the bonds, a weaker bond will show a longer wavelength vibration and hence a lower frequency.
The diffuse pi system is removed from 12 by saturating its exo double bond. The sigma-bonded framework is less diffuse and, critically, less able to undertake in orbital mixing.
This orbital mixing serves to weaken the C-Cl bond by having the pi system donate electron density into one of its antibonding orbitals. By retrospection, at the images in Part 1 above, the orbitals that most fit this theory is donation from the HOMO into the LUMO+1.
Schematically, it can be shown as donation from a pi cloud into the C-Cl sigma* orbital as shown below.
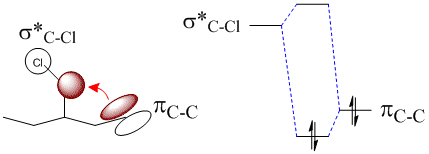
The donation of electron density into the C-Cl sigma* orbital gives the C-Cl bond more antibonding character and so weakens it.
The stretching frequencies of molecule 12 and its monohydrogenated derivative 12i are compared below. They were calculated using b3lyp/6-31G(d,p.
| Stretch | 12 cm-1 | 12i cm-1 |
|---|---|---|
| C-Cl | 770.8 | 774.99 |
| Endo C=C | 1757.42 | 1758.08 |
| Exo C=C | 1736.96 | N/A |
The stretches shown above concur with the theory that the C-Cl bond is weakened by 4.5cm-1 (around 12.9kcal/mol)due to orbital overlap. As the pi bond is donating electrons, it effectively becomes less bonding and so a weakening in the double bond is expected. The vibrations show this weakening to be around 1.7cm-1 (4.0kcal/mol).
Structure based Mini project using DFT-based Molecular orbital method
Assigning regioisomers in "Click Chemistry"
"Click-Chemistry" is the name of a chemical philosophy which involves reactions from small 'highly-sprung' starting materials that maximises atom economy and ease of synthesis with a high degree of specificity[10].
A recent addition to the roster of click chemistry is a catalysed triazole reaction. Interestingly, Copper(II) and Ruthenium (II) give different isomers as shown below.
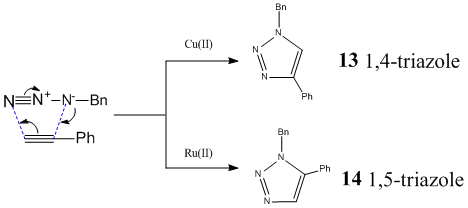
As these are assymetrical conformers, they can be analysed by 13NMR. A calculation using MM2 to optimise the geometry was run before using MOPAC/PM6 to afford the mpw1pw91/6-31(d,p) data for 13 and 14 .The geometry was further optimised using mpw1pw91/6-31g(d,p) and SCAN. This geometry was used to calculate the NMR values tabulated below alongside with the values found in the literature[11][12]. The calculated NMR data can be found here for 13 DOI:10042/to-4352 and 14 DOI:10042/to-4351
| 14 | ||
| Shift/ppm | Assignment | Literature/ppm |
| 52.307 | 6 | 52 |
| 123.404 | 12 | Multiplet |
| 123.671 | 8 | Multiplet |
| 124.482 | 10 | Multiplet |
| 124.695 | 18 | Multiplet |
| 125.191 | 16 | Multiplet |
| 125.474 | 11 | Multiplet |
| 125.49 | 9 | Multiplet |
| 125.616 | 14 | Multiplet |
| 125.876 | 15 | Multiplet |
| 126.252 | 13 | Multiplet |
| 128.621 | 1 | 433.5 |
| 134.096 | 7 | 136 |
| 136.669 | 2 | 138.5 |
| 13 | ||
| Shift/ppm | Assignment | Literature/ppm |
| 54.783 | 6 | 54 |
| 117.028 | 2 | 119.5 |
| 121.285 | 18 | 126 |
| 121.845 | 14 | 126 |
| 124.134 | 16 | Multiplet |
| 124.241 | 8 | Multiplet |
| 124.533 | 12 | Multiplet |
| 124.812 | 10 and 11 | Multiplet |
| 124.969 | 17 | Multiplet |
| 125.212 | 15 | Multiplet |
| 125.554 | 9 | Multiplet |
| 127.273 | 13 | 131 |
| 133.221 | 7 | 135 |
| 145.09 | 1 | 148 |
Conclusion: Very good correlation with literature values. High chance of being correctly assigned originally!
References
- ↑ P.J. Wilson and J.H. Wells, Chem. Rev., 1944, 34(1), 1: DOI:10.1021/cr60107a001
- ↑ Woodward, R. B.; Hoffmann, R., The Conservation of Orbital Symmetry; Academic Press: New York, 1970
- ↑ R. B. Woodward, R. Hoffmann, J. Am. Chem. Soc., 1965, 81, pp395DOI:10.1021/ja01080a054
- ↑ R. B. Woodward, R. Hoffmann, J. Am. Chem. Soc., 1965, 81, pp2511DOI:10.1021/ja01089a050
- ↑ Henry Rzepa, Organic Pericyclic Reactions, Imperial College London, 2009, Accessed 05/03/2010
- ↑ D. Skála and J. Hanika, Pet.Coal, 45, 2003, pp105
- ↑ Accessed 05/03/2010 http://en.wikibooks.org/wiki/Computational_chemistry/Molecular_mechanics
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- ↑ W.F. Maier and P.v.R. Schleyer, J. Am. Chem. Soc., 1981, 103, pp.1891: DOI:10.1021/ja00398a003
- ↑ Hartmuth C. Kolb, M. G. Finn, K. Barry Sharpless, Angew. Chem. Int. Ed. 2001, 40, 2004, pp.2021 DOI:<2004::AID-ANIE2004>3.0.CO;2-5 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5
- ↑ Ionic Polymer Supported Copper(I): A Reusable Catalyst for Huisgen's 1,3-Dipolar Cycloaddition, Uthaiwan Sirion, Yu Jin Bae, Byoung Se Lee, Dae Yoon Chi, Synlett 2008 2326-2330 DOI:10.1055/s-2008-1078245
- ↑ Li Zhang,Xinguo Chen, Peng Xue, Herman H. Y. Sun, Ian D. Williams, K. Barry Sharpless, Valery V. Fokin, and Guochen Jia; J. Am. Chem. Soc., 2005, 127 (46), pp15998 DOI:10.1021/ja054114s