Rep:Mod:s j hines org mp
Structure Based Mini Project Using DFT-based Molecular Orbital Methods
Investigating the Regioselectivity of the Baeyer-Villiger Reaction
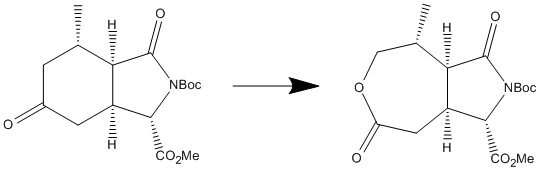
The scheme below is an example of a regioselective Baeyer-Villiger reaction. It is an important part of the synthesis of '(-)-kainic acid' (a 'neuropharmacological tool'). This reaction invloves a ketone being oxidised to make an ester. As is true in the case of the below reaction, a common reagent for this reaction is m-CPBA.
|
|
|
A number of computational techniques can be, and indeed were, used to demonstrate the regioselectivity of the above reaction.
Prediction of IR Spectra
A similar method to that of the section 'Regioselective Addition of Dichlorocarbene' was used in order to predict the vibrational spectrum of each of the above compounds to enable comparison between them, and the values in the literature. The molecules were constructed, then their geometry minimised using the density functional molecular orbital method(B3LYP/6-31G(d)), having been pre-optimised by the simple 'MM2' molecular mechanics method. This calculation, which, in each case, took approximately nine hours to complete, also allowed analysis of stretching vibrations within each molecule, in particular the C=O stretches, as it is this group (as a ketone) which is expected to be oxidised to an ester group. There are four carbonyl groups in each molecule, so it is expected that there will be four absorptions in the within the appropriate range, each one correponding to the vibrational frequency of a particular C=O within the molecule. For the starting compound, the predicted IR frequencies from the molecular orbital method of calculation were visualised, and found to be: 1838 cm-1 for the C=O situated above the NBoc group on the five membered ring (as shown on the 2D reaction scheme), 1826 cm-1 for the C=O as part of the CO2Me group, 1823 cm-1 for the C=O of the ketone (the expected site of oxidation), and 1777 cm-1 for the C=O as part of the Boc group. The corresponding C=O stretches for the product molecule were: 1852 cm-1, 1834 cm-1, 1809 cm-1, and 1780 cm-1, respectively. It was noticed that the intensity of the signal corresponding to the C=O of the site of oxidation, increased significantly (by around 80%) going to the starting molecule to the product. This maybe attributed to the fact that the C=O group is now attached to a seven-membered ring rather than a six-membered ring in the starting material. These were then compared to the IR frequencies in the literature[1]. Also four stretching frequencies were found here for each compound. It is unspecified which carbonyl groups to which these stretches belong, however these were 1789 cm-1, 1746 cm-1, 1729 cm-1 and 1713 cm-1 for the starting material, and 1780 cm-1, 1755 cm-1, 1748 cm-1 and 1723 cm-1 for the product. It is seen that the values in the literature are significantly lower than that found to be the case of the predicted IR values using the molecular orbital method here. There are a number of possible reasons for this 40 cm-1 - 100 cm-1 discrepancy. It is an indication that the molecules entered for the calculation in this computational experiment are different from the molecules reported in the literature.[1] The C=O stretching frequencies found using the molecular orbital method in this experiment are higher than what might usually be expected for this group. This could either be down to the wrong assignment of the stereochemistry of the molecule in the literature or the conformation investigated in this experiment is not entirely correct. The other option, of course, is that the predictions for these vibrations are simply inaccurate. This technique would have been useful for futher analysis of the compounds and reaction if the literature was able to assign particular C=O stretches to each particular C=O contained within each molecule - the changes in frequency between reactants and products. The fact that there are four stretching frequencies for the C=O group in both the predicted IR spetra and the reported spectra for the product, shows that they are still present, providing evidence that the mechanism discussed is still valid.
Prediction of 13C NMR Sprectra
In order to predict the 13C NMR sprectra of the two above compounds, each one needed to have its geometry optimised. Following a simple optimisation through the 'MM2' molecular mechanics method, the density functional molecular orbital method, mpw1pw91/6-31G(d,p) was applied and the calculation run. This took around four and a half hours to carry out. Once this had completed, and NMR chemical shift calculation was run.
The data produced with this model was as follows:
Starting material δ (CDCl3) 205.5, 169.8, 163.2, 148.0, 82.1, 64.6, 54.6,49.0, 45.2, 38.7, 33.1, 30.5, 29.5, 25.5, 25.4, 22.2. (16 signals)
Product δ (CDCl3) 167.7, 167.1, 164.0, 146.8, 82.7, 68.8, 66.9, 55.0, 51.2, 34.8, 33.7, 30.6, 30.3, 25.1, 25.4, 16.5. (16 signals)
The corresponding data in the literature [1] is:
Starting Material δ (CDCl3) 207.95, 172.75, 170.34, 149.41, 84.08, 62.65, 52.73, 47.09, 44.97, 42.05, 34.54, 29.34, 27.78, 20.30. (14 signals)
Product δ (CDCl3) 173.6, 171.5, 170.6, 149.1, 83.9, 66.3, 62.6, 52.6, 52.1, 46.3, 35.1, 33.3, 32.9, 27.8, 13.6. (15 signals)
There were also found to be differences in the 13C NMR data between those calculated through this computational molecular orbital method and those reported in the literature.[1] The predicted spectra for each compound showed 16 signals on each, however the literature reports only 14 signals for the starting material and 15 for the product. Again this may indicate different conformations of the molecules pictured above to the ones that might have been mistakenly assigned as these in the literature. It can be seen that there are many similar correponding values that can be paired up between the results of the molecular orbital type calculations and the literature. For the starting material, corresponding signals can be seen almost all the way through (the difference is small between them e.g. no more than ~ 2 ppm), except for a difference of ~ 7 ppm at one point (170 ppm compared to 163.2 ppm). The extra signals in the literature occur at the low ppm end, where the literature only has a signal at 20.30 ppm compared to three signals for the experimental calculation at 25.5 ppm, 25.4 ppm and 22.2 ppm. The trend between the values of the product is not so good. The majority of the literature readings are above those predicted in the calculations by 3 ppm - 6 ppm. The extra signal again occurs at the lower ppm end. In the literature there is one signal at 13.6 ppm, whereas in the calculated predicted spectrum there are signals as 25.1 ppm and 16.5 ppm. These results described here once again indicate that the molecules that were analysed in these molecular orbital calculations are not exactly the same as the molecules that were found or described in the literature (the other possible option being that the predicted spectra does not give an accurate picture as to what the spectrum may actual look like if carried out on these two compounds).[1]
Mechanism of Reaction
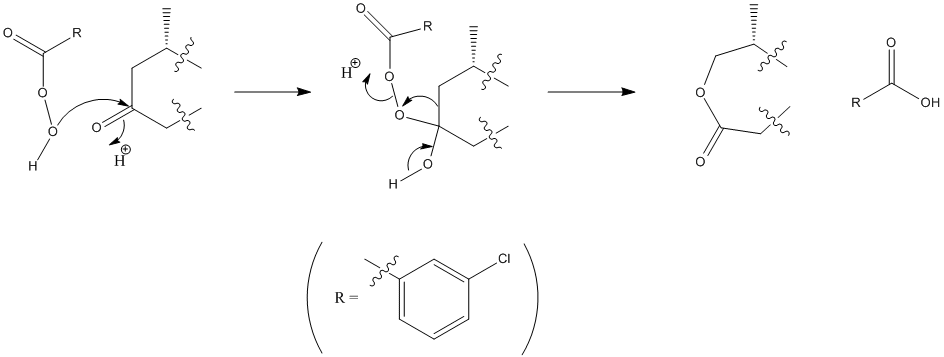
A reason for this reaction being so regioselective could be the nature of the Baeyer-Villiger reaction itself. The m-CPBA in this reaction reacts with a ketone to produce a cyclic ester. The reason for the regioselectivity of the C=O that is oxidised in this reaction is down to a concept called 'migratory aptitude'.[3] or the relative tendency for a group to take part in rearrangement. Here, there has been a rearrangement in order to insert the oxygen that is now part of a seven-membered ring. The alkyl group as part of the six-membered ring of the starting material is able to stabilise positive charge most effectively, thus it has the highest tendency to migrate. This is shown in the second stage of the mechanism above. Due to the attachment of atoms in the molecule adjacent to the other carbonyl groups, these are not able to be oxidised in the same way using the m-CPBA - rearrangment/migration in that manner is disfavoured, thus rationalising the high regioselectivity of this reaction.
References
- ↑ J. Clayden et al., Tetrahedron, 2002, 58, 4727-4733
- ↑ A. Baeyer & V. Villiger, Chem. Ber., 1899, 32, 3625-3633
- ↑ Alan D. McNaught & Andrew Wilkinson, IUPAC Compendium of Chemical Terminology, 2e, 1997

