Rep:Mod:s j hines inorg mp
Computational Inorganic Chemistry Laboratory - Mini Project - 'Fuels of the Future'
|
In this section, the molecule 'ammonia borane' will be considered. We are quickly running out of sustainable fuels, so alternatives to oil and natural gas are needed urgently. It has been decided that far the most like source is going to by from hydrogen. The main problem at the moment is the need of a way to store this fuel effectively and safely[1][2]. The fairly high stability along with high perecentage hydrogen content of ammonia borane has caused this substance to be sounded as a possible solution to the problem mentioned.[3][4] Here, the molecule itself will be examined to find out a bit more about its properties and possible use then also come to some conclusions as to the suitability of this substance. The 3D representation of the ammonia borane molecule shown right is the fully optimised one evolved from stage 4. |
|
The ammonia borane molecule is 'isoelectronic' to the ethane molecule, however there are important differences between the two molecules. There is a dative bond in the ammonia borane molecule - the lone pair on the nitrogen to the vacant p-orbitals of the boron, where as in ethane, the C-C bond is just a covalent bond with one electon from each carbon. The first stage was to optimise the structure. Ethane was included in this to allow comparison betweem the two molecules. It was decided that the optimisation of the each molecule should take place in four different stages, each time increasing the accuracy and intensity of the calculation, so that in the end, an accurate representation of the molecule could be analysed.
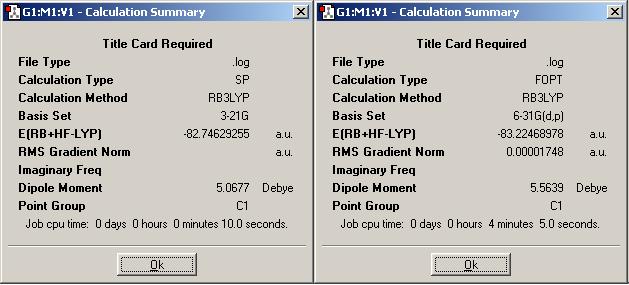
The summary snapshots shown (above for ammonia borane (DOI:10042/to-1346 and DOI:10042/to-1347 ), below for ethane (DOI:10042/to-1351 and DOI:10042/to-1352 )) here are for the first 2 stages of the optimisation. The first stage involved the B3LYP/3-21G method, then the second stage was B3LYP/6-31G(d,p).
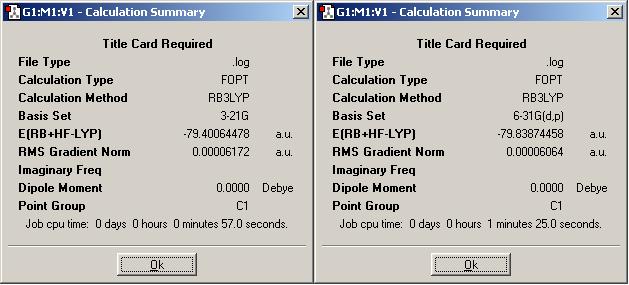

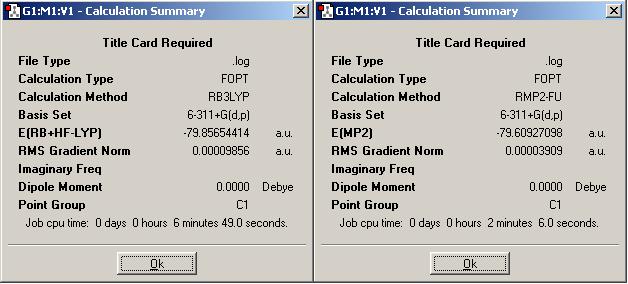
Here are the summaries of stages 3 and 4 (above for ammonia borane (DOI:10042/to-1348 and DOI:10042/to-1349 ), below for ethane (DOI:10042/to-1353 and DOI:10042/to-1354 )). Stage 3 used the B3LYP/6-311+G(d,p), and fourth stage used the most accurate method of MP2/6-311+G(d,p).
From the optimised structure, it is possible to tell that the ammonia borane (and ethane) molecule is staggered. Examining the output has given geometrical coordinates of the molecule (angstroms): Boron at (-0.927898, 0.000000, 0.000000), hydrogens at (-1.236039, -0.283890, 1.133504), (-1.236039, -0.839699, -0.812608), (-1.236031, 1.123590, -0.320896), (1.091239, 0.681192, 0.659207), (1.091240, 0.230299, -0.919531) and (1.091251, -0.911483, 0.260323), and a nitrogen at (0.724839, 0.000000, 0.000000).
Bond angles and lengths were also found: N-H 1.01628 Å N-B 1.65274 Å B-H 1.20846 Å H-B-H 113.73299 ° H-N-H 107.75951 ° H-B-N 104.77278 ° H-N-B 111.13354 °
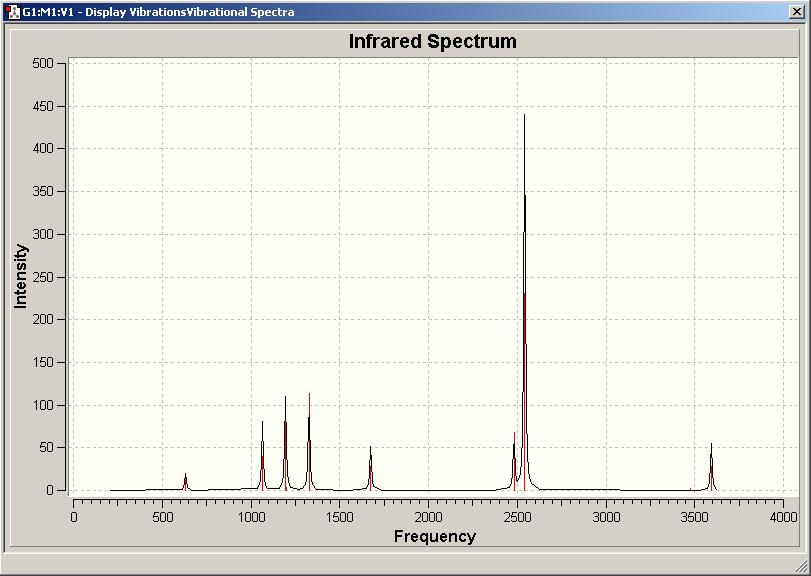
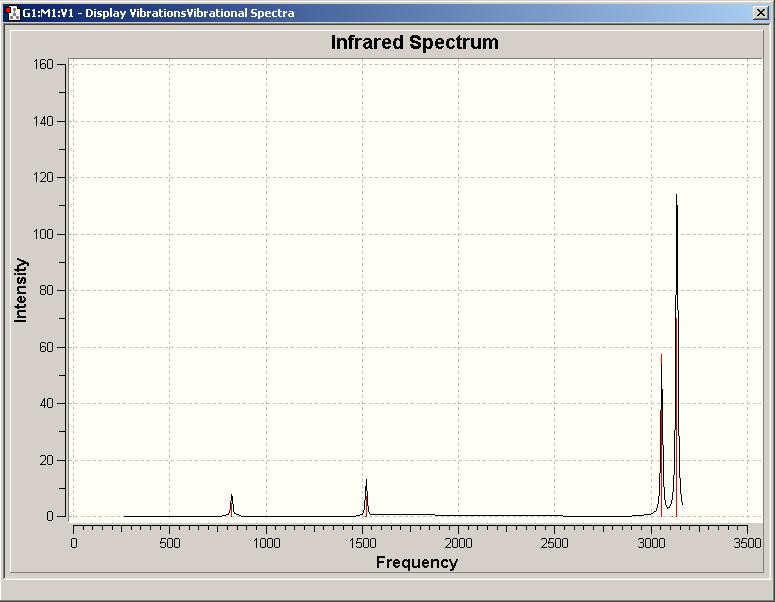
The optimised structures of ammonia borane and ethane were taken from stage 4, and analysed to produce predicted vibrations/IR spectra for each molecule. These are pictured below (top is ammonia borane(DOI:10042/to-1350 ), bottom is ethane(DOI:10042/to-1355 )).
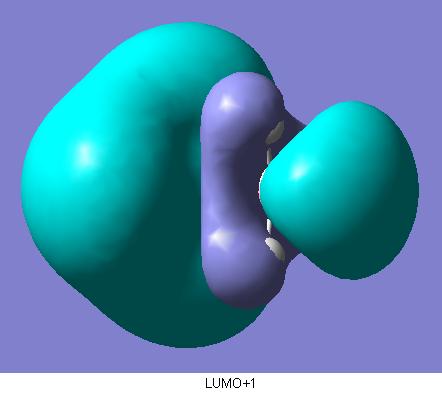
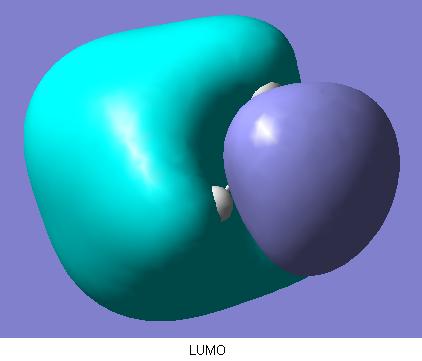
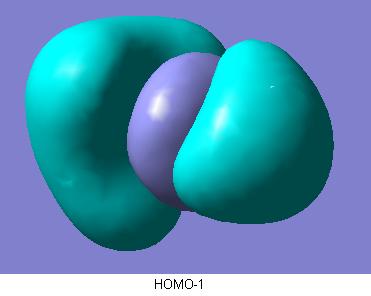
The final part of this section involved the calculated quantative prediction of the molecular orbitals of ammonia borane. These are shown below.

References
- ↑ T. Autrey et al., Energy Environ. Sci., 2008, 1, 156–160
- ↑ F.H. Stephens et al., Angew. Chem. Int. Ed. 2007, 46, 746 –749
- ↑ http://www.rsc.org/Publishing/ChemScience/Volume/2008/08/Borane_fuels.asp
- ↑ F.H. Stephens et al., Dalton Trans., 2007, 2613–2626
