Rep:Mod:rr12102
Transition states and reactivity
The objectives of the following calculations are to determine the preferred mechanism of a given reaction through characterising the potential energy surface of the molecules. Low energy minima, corresponding to the energetically favoured ground state conformations of products and reactants can be found through structural optimisations. The transition states correspond to high energy local maxima in the potential energy surface, and can be characterised from a guess structure by finding the closest maxima. This information can then be used to suggest which reaction mechanism is most likely in a given case, along with giving relative energies of products, reactants and transition states.
Gaussian09 was used to run all the calculations in this wiki, as was Gaussview5 to create input files and visualise output files.
This link is a wiki page containing log files, item tables and low frequency lines (as appropriate) for each of the calculations run during the lab. Specific sections of the page are also linked to throughout this wiki page, when the calculation concerned is discussed.
The Cope rearrangement
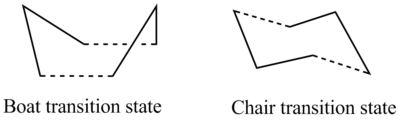
The Cope rearrangement is a concerted [3,3]-sigmatropic shift, which can go via either a boat or a chair transition state, although the boat transition state is slightly less stable. [1]

The approach taken to confirm this computationally will be to first optimise
Optimising the reactants and products
Optimisation of six conformers at the HF/3-21G level
Initially six conformers were drawn and optimised, in order to get an overview of the effect on the energy of the molecule of different conformations. Three conformers where the central four carbon atoms have a gauche formation and three where they have an anti formation were created. The six conformers were drawn with all six carbon atoms in the same plane.
The method for these optimisations was the Hartree Fock, used with the basis set 3-21G. Each structure was symmetrised after optimisation to give the point groups shown in the table.
The table below shows the conformers as drawn, after clean-up and after optimisation. The summary tables and log files for all six optimisations are available here.
All of the optimised structures show large deviations from the structures drawn. None of the conformers remained planar, and three lost all symmetry to become C1 molecules, including the conformation with the lowest energy. The energies and relative energies correspond exactly with the molecules seen in appendix one of the instruction wiki.
Discussion of relative energies
The lowest energy conformer has a gauche linkage, which is suprising as shorter alkanes generally have a lowest energy form which is entirely trans. The trans conformation is favoured by interactions between pairs of antiperiplanar C-C bonds (where the empty σ* of one bond interacts favourably with the doubly occupied σ of another to bring the energy of that electron pair down slightly); the gauche conformations only have these interactions between C-H bonds and between C-H and C-C bonds, neither of which are as favourable.
However, the next two conformations are both anti, and all three of the lowest energy conformations are very close in energy. The three highest energy conformations seem to be less stable due to disfavourable interactions, such as Pauli repulsion between pairs of filled bond orbitals, and between pairs of hydrogen atoms. For example in the Gauche 5 isomer there is a pair of hydrogen atoms 2.2Å apart which could be destabilising the conformer slightly.
Another effect which could explain the stability of the gauche 3 conformer is favourable interactions between π*C=C orbitals and σC-C (or vice versa) which are possibly enhanced by that particular conformation.
| Isomer | Relative energy/ Kcal mol-1 | Dihedral angle | C-C->C-C APP pairs | C-C->C-H APP pairs | C-H->C-H APP pairs |
|---|---|---|---|---|---|
| Gauche 3, C1 | 0 | 68° | 0 | 4 | 5 |
| Anti 1, C2 | 0.04 | 177° | 1 | 2 | 6 |
| Anti 2, Ci | 0.08 | 180° | 1 | 2 | 6 |
| Gauche 2, C2 | 0.62 | 64° | 0 | 4 | 5 |
| Anti 4, C1 | 1.06 | 178° | 1 | 3 | 5 |
| Gauche 5, C1 | 1.91 | 71° | 0 | 5 | 4 |
Optimisation of the Anti 2 conformer at the B3LYP/6-31G* level
Here is a link to the log file and item table for this optimisation, and the B3LYP/6-31G* optimisation of the gauche 5 conformer, done to compare relative energies between conformers using the two methods.
The anti 2 conformer was optimised at a higher level using the B3LYP DFT functional with the 6-31G* basis set.
The table below shows a comparison between some key information in the two calculations. The geometry of the structure remained very similar, however all the bond lengths decreased slightly in the second optimisation. The absolute energies are very different, with the DFT optimisation giving a much lower energy for the conformer, however the relative energies between conformers (optimised with the same method) were only slightly depressed when using DFT compared to HF methods.
Frequency calculation for the Anti 2 conformer at the B3LYP/6-31G* level
Here is a link to the log file and low frequencies table for this calculation.
A frequency calculation was run on the anti 2 conformer, again using the DFT method. This allows us to check that the optimisation has indeed reached a minimum in the potential energy surface, view a calculated IR spectrum and collect thermochemical data which is comparable to experimental values.
All of the frequencies generated are real, not negative which confirms that the optimisation was successful in finding a minimum. The infrared spectrum is included below.
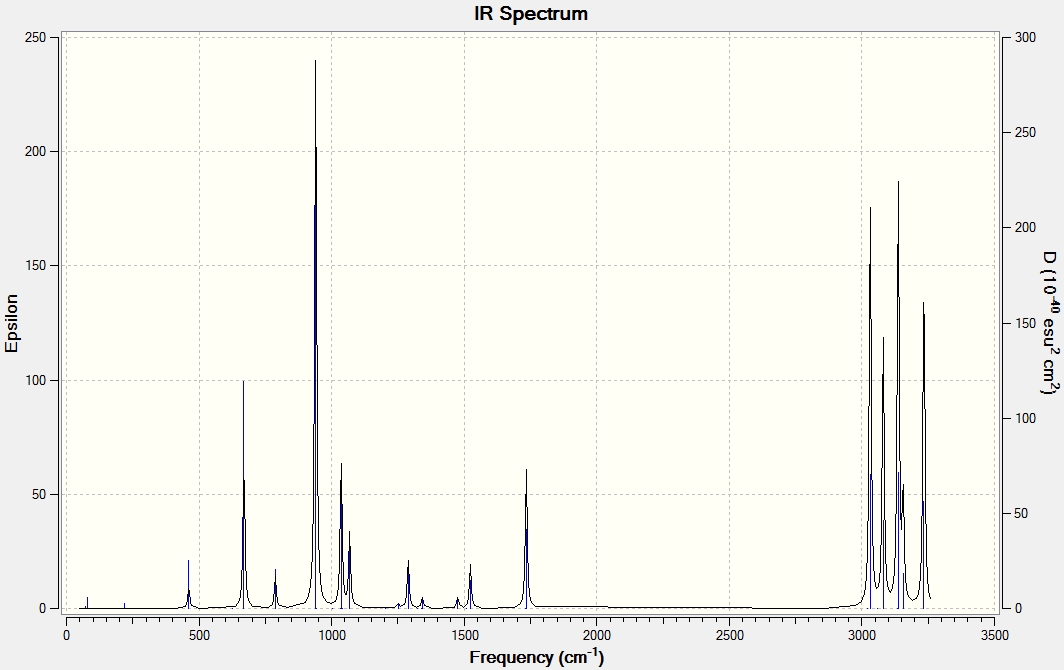
Thermochemical analysis of the Anti 2 conformer
Sum of electronic and zero-point Energies: -234.469212 Sum of electronic and thermal Energies: -234.461856 Sum of electronic and thermal Enthalpies: -234.460912 Sum of electronic and thermal Free Energies: -234.500821
Optimising the transition states
The chair and boat transition states were optimised using several different methods which are detailed below. Most of these methods made use of guess transition state structures which were formed using pairs of HF/3-21G optimised ally fragments. The log file and item table for that calculation can be found here.
HF/3-21G optimisation of the chair transition state
The log file and item table for this calculation can be found here.
The optimisation was run using the job type optimise to a TS (Berny). The force constants were calculated once and the keyword Opt=NoEigen was used to allow multiple imaginary frequencies during the calculation.
Frequency analysis
The log file and low frequency lines for this calculation can be found here.
The HF/3-21G method was again used to run a frequency job on the optimised structure. This allowed confirmation that the structure was indeed a transition state as there was one imaginary frequency at -818cm-1.
Insert animation of vibration
Frozen coordinate optimisation of the chair transition state
The bond length of the made/broken bonds in the guess transition state structure was frozen at 2.2Å using the Opt=ModRedundant keyword, thereby holding the molecule at a particular reaction coordinate. A normal optimisation to a minimum using the HF/3-21G method was then performed to fully relax the molecule at that reaction coordinate.
From this relaxed structure it was possible to optimise to a transition state without calculating the force constant matrix (the Hessian) instead the Opt=ModRedundant keyword was again used, this time to allow the calculation to optimise along the bond being created in the rearrangement.
The log file and item table for the initial optimisation is available here.
The log file and item table for the transition state optimisation is available here.
check the bond forming/bond breaking bond lengths and compare the structure to the one you optimized in section (b).
QST2 optimisation of the boat transition state
This method involves inputting the optimised reactant and product into Gaussview and then calculating a transition structure from them. The HF/3-21G method was used again.
For QST2 to give a particular transition structure (in this case we wanted to optimise to the boat structure) the products and reactants need to be in a conformation close to that transition state.
When the anti 2 conformer was used as it had been optimised the calculation did not give a boat transition structure, instead it gave a structure similar to the chair transition state but more dissociated. This is because the calculation simply moved the top allyl fragment to the left until the molecule reached the product conformation.
The conformer was modified by changing the central dihedral angle to 0° and the 2-3-4 and 3-4-5 bond angles to 100°. This allowed the calculation to find the boat transition state desired. The optimisation was confirmed to have converged by both the item table and the lone imaginary frequency vibration at -840cm-1
| Product | Reactant | Transition state |
|---|---|---|
 |
 |
|
 |
 |
|
The item tables, low frequency lines and log files for both calculations are here.
IRC calculation on the chair transition state
The log file is available here.
By inspection alone it is hard to guess or prove which conformer of cyclohexadiene a particular transition leads to. Doing an intrinsic reaction coordinate calculation allows the program to move in small steps from the transition state geometry downwards in energy along the steepest energy gradient at any point, once the calculation reaches a local minima this is the structure it outputs, which should correspond to the relevant conformer formed by the reaction.
The method used was again HF/3-21G, and the calculation was only run in the forward direction.
The conformer formed was the gauche C2 conformer, gauche 2.
DFT optimisations and frequency calculations
The log files item tables and low frequency lines are available here.
| HF/3-21G Energy/Hartrees | B3LYP/6-31G Energy/Hartrees | HF/3-21G Relative energy/Kcalmol-1 | B3LYP/6-31G Relative energy/Kcalmol-1 | |
|---|---|---|---|---|
| Chair | -231.61932223 | -234.55698303 | 45.94184779 | 34.33714815 |
| Boat | -231.60280235 | -234.54309304 | 56.30822117 | 43.05324189 |
| Anti 2 | -231.69253528 | -234.61170280 |
The Diels-Alder cycloaddition
The Diels-Alder cycloaddition is a pericyclic reaction between a diene with four π electrons and a dieneophlie with two π electrons. If we assume the reaction is thermal this means it must proceed suprafacially via a Huckel transition state. This means that the HOMO of one reactant must have the same symmetry (with repect to the plane of symmetry which is perpendicular to the plane of the molecule) as the LUMO of the other to get sufficient orbital overlap for a reaction.
All the calculations detailed below were performed using the AM1 semi-empirical molecular orbital method.
The prototype reaction
The simplest Diels-Alder reaction is that of ethylene and butadiene. This reaction will be briefly investigated to illustrate the symmetry considerations which apply.
Optimising cis butadiene
The log file and item table may be found here.
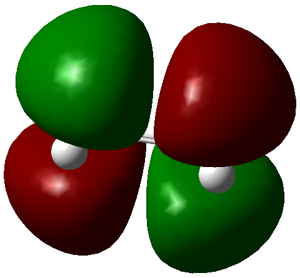
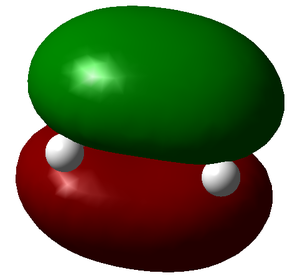
| LUMO | HOMO |
|---|---|
 |
|
 |
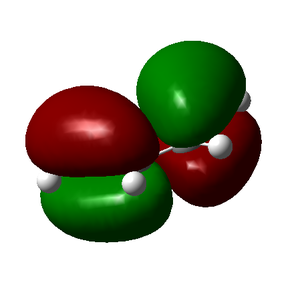
|
Here we can see that the LUMO of ethylene is antisymmetric and the HOMO symmetric, and vice versa for butadiene. This means the reaction is allowed and does occur if it is also energetically favourable.
Optimising the transition state
The log files and may be found here.
The transition state was calculated simply using a guess structure then optimising to a maximum. The bond lengths used for the new bonds were 2.2Å. The convergence was confirmed by the single imaginary frequency at -956cm-1, which corresponds to the Diels-Alder reaction when animated. Both bonds form simultaneously.
| HOMO (antisymmetric) | LUMO (symmetric) |
|---|---|
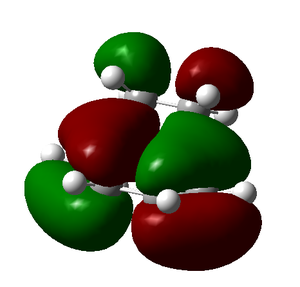 |
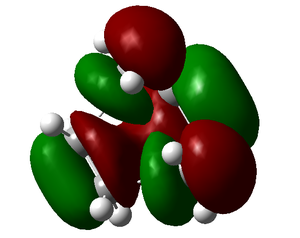
|
The HOMO is antisymmetric and has been formed from the HOMO of the butadiene molecule and the LUMO of the ethylene molecule.
| Bond | length/Å |
|---|---|
| Van der waals radius | 1.70 |
| sp2 | 1.34 |
| sp3 | 1.54 |
| 3-9 (and 2-12) | 2.12 |
| 1-4 | 1.40 |
The new bond length is less than twice the van der waals radius of carbon.
Diels-Alder regiochemistry
Optimising the reactants and products
The log files and may be found here.
Optimising the transition states
The log files and may be found here.
| Isomer | E/Hartrees | Relative E/Kcalmol-1 |
|---|---|---|
| Endo | -0.05150468 | 0 |
| Exo | -0.05041985 | 0.68 |
The endo transition sate was found to be slightly favoured compared to the exo transition state.
References
- ↑ Olaf Wiest, Kersey A. Black, and K. N. Houk, J. Am. Chem. Soc. 1994, 116, 10336-10337