Rep:Mod:rom3112
Module 1: Structure and Spectroscopy
The Hydrogenation of Cyclopentadiene Dimer
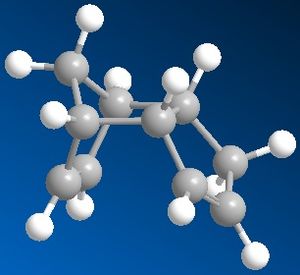
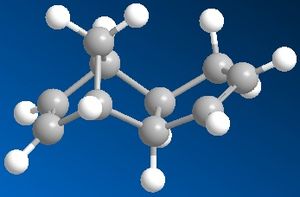
The dimerisation of cyclopentadiene results in the production of mainly the endo isomer over the exo isomer. From modelling the two isomers in Chem3D, the results show that thermodynamically the exo isomer is more stable and therefore could be predicted as the favoured product. This was shown by drawing the structures in Chem3D and running a molecular mechanical optimisation on both isomers to gain geometries with the lowest energies for comparison. The results showed that the exo isomer had an overall energy of 31.88 Kcal/mol, which was lower than that of the endo isomer at 34.02 Kcal/mol. Thermodynamically, a molecule with a lower energy is more stable and therefore favoured over the molecule with the higher energy.
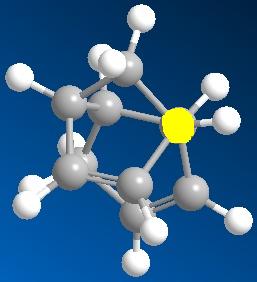

On a closer look at the components of the energy, it is seen that the main diffference in energy between the isomers is caused by the difference in contribution of torsional strain; The value changing from 7.66 to 9.50 Kcal/mol from the exo to the endo isomer. This means that there is more torsional strain in the endo isomer, making it less stable than the exo isomer. This can be seen in the following images of Newman projections down the highlighted bonds of the isomer. The Newman projection from the endo isomer shows a staggered conformation but with the largest substituents from both of the rings in syn-clinal (60 degrees) to each other, whereas the exo isomer shows the staggered conformation with the largest substituents anti-periplanar (180 degrees) to each other. The anti-perplanar conformation gives the least steric hindrance, and hence less torsional strain, making the mollecule more stable. These results so far show that the exo isomer should be the favoured isomer and therefore be the main product.
However, the molecular mechanic approach doesn't include consideration into electronics and orbital interactions.
To explain the reason why the endo product is favoured we have to look at the mechanism of reaction. The dimerisation of cyclopentadiene occurs via a diels-alder reaction, involving cyclopentadiene acting as both diene and dienophile. To form the endo product , the dienophile approachs the diene with its substituent directed towards the diene, this results in a transition state where the p-orbitals of the diene overlap in a favourable way with the p-orbitals on the substituent of the dienophile. There is no overlap between the p-orbitals of the substituent of the dienophile and the p-orbitals of the diene in the exo transition state as the dienophile approachs the diene with its substituents directed away.
This concludes that even thought the exo isomer is the thermodynamically more stable isomer, the endo isomer is the kinetically more stable isomer so the reaction is kinetically controlled.
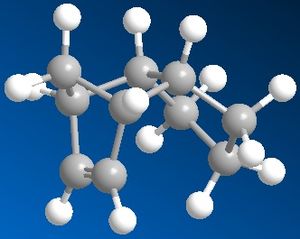
From analysing the structures of the hydrogenated exo isomer, the results showed that 4 was more thermodynamically stable than 3. 4 had an overall energy of 31.15 Kcal/mol compared to 3 with 35.93 Kcal/mol. The main component causing this difference was the bend, which is the amount that the bond angles deviate from the ideal value. The values changed from 18.86 to 14.51 Kcal/mol from 3 to 4. The stretching values, which show the amount by which the bonds are distorted form the ideal value, went from 1.23 to 1.10 Kcal/mol from 3 to 4, showing little difference. The torsion values were very similar with 12.25 Kcal/mol for 3 and 12.50 Kcal/mol for 4, this shows there is slightly more steric hindrance in the geometry of 4. There was a difference of 1.24 Kcal/mol for the 1,4 van der waals contributions between 3 and 4, destabilising 3 more than 4, but there was a difference of 0.51 Kcal/mol for the non-1,4 van der waals contributions, stabilising 3 more than 4.
This concludes that the thermodynamic product is 4.
References
http://www.enc.edu/~timothy.t.wooster/courses/CH322/Lab/3-21%20The%20Diels%20Alder%20reaction.pdf
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
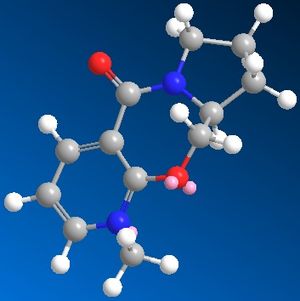
5 was constructed on Chem3D and the geometry was optimized giving the overall energy of 67.82 kcal/mol and a dihedral angle of 23° bewteen the carbonyl and the aromatic ring. The carbonyl was moved to different positions and the molecule was optimisation was repeated and the following results were obtained:
Dihedral angle before optimisation: -63°, -30°, 56°, 101°
Dihedral angle after optimisation: -110°, 13°, 17°, 21°
Energy after optimisation (kcal/mol): 193.86, 67.92, 67.66, 67.19
These results show that there is mainly one position (around 20°) where the carbonyl group will lie with respect to the aromatic ring, this is the global minimum for the geometry, the other value shown (-110°) is a local minimum as the energy is higher than the global minimum. This shows that the carbonyl group is slightly above the ring.
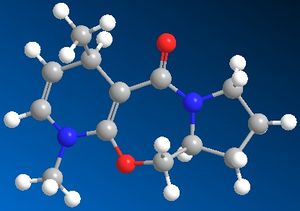
Upon addition of MeMgI, 6 is produced with the methy group attacking as a nucleophile para to the nitrogen instead of ortho. When constructed, the optimized energy of the molecule is 28.92kcal/mol. The mechanism of reaction proceeds through a transition state in which the lewis acidic Mg coordinates to the carbonyl, due to its lone pairs. The methyl group can then attack the aromatic ring para to the nitrogen. As the carbonyl group is above the plane of the ring, the methyl group will attack from above the plane of the ring hence giving the geometry shown.
7 was constructed on Chem3D and the geometry was optimized giving the overall energy as 39.54kcal/mol and the dihedral angle between the carbonyl and the aromatic ring as 78°. The carbonyl was moved to different angles with respect to the aromatic ring and the optimisation was repeated and the following results were obtained:
Dihedral angle before optimisation: -47°, 88°, 78°, -136°
Dihedral angle after optimisation: 29°, 29°, 82°, -110°
Energy after optimisation (kcal/mol): 38.10, 38.10, 39.16, 151.16
This shows there are two positions with almost the same energy but that 29° above the ring gives the global minimum energy.
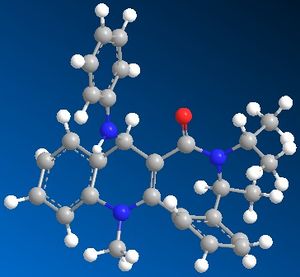
Upon addition of PhNH2, 8 is produced. When constructed, the optimized energy of the molecule is 13.27kcal/mol. The carbonyl group H-bonds to one of the hydrogens on the reactant, this puts the reactant above the ring so when the nitrogen attacks the aromatic ring it does so from above the ring and hence ends up in the stereochemistry shown (above the ring).
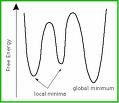
The diagram shows what is meant by local and global minima (the x axis should read dihedral angle). If the angle was to be put on the slope towards the local minimum, the calculation would result in giving the local minimum as the lowest energy possible, which would be incorrect as the lowest possible energy is the global minimum. (the graph isn't factual for the above molecules).
Image from: www.cryst.bbk.ac.uk
References
1. Regio- and Stereoselective Control in the Addition of Grignard Reagents to the Pyridine Ring System. Arthur G. Schultz, Lawrence Flood and James P. Springer
2. Preparation of axially chiral quinolinium salts related to NAD+ models: new investigations of these biomimetic models as chiral amide-transferring agents. Stephane Leleu, Cyril Papamical, Francis Marsais, Georges Dupas and Vincent Levacher
Stereochemsitry and Reactivity of an Intermediate in the Synthesis of Taxol
10 |
Both antropisomers were constructed on Chem3D and the geometries were optimised returning the results of 44.29kcal/mol for 11 and 120.59kcal/mol for 10. This shows that 11 is thermodynamically more stable than 10. The main contribution to the difference in these energies is the bend component which varies 66.10kcal/mol from 11 to 10.
11 |
According to Bredt's Rule, an alkene at the bridging terminal of a bridged ring system cannot exist. This is due to the pi-orbitals of the bridging carbon being twisted to an angle not accessible to the neighbouring carbons pi-orbitals (see diagram)
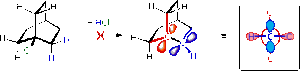
This rule can be exempt for large ring systems as the flexibility within the system can result in good overlap of the pi-orbitals. The isomers show cyclo-octene rings, which are the smallest ring systems that can show cis and trans cycloalkenes, any smaller and only the cis isomer can be seen. From the images it is seen that the isomers take on the cis-conformation, which is thermodynamically more stable than the trans. This results in a stable alkene which will have low reactivity.
Images taken from: http://www.cem.msu.edu/~reusch/VirtualText/alhalrx3.htm
References
1. THE FIRST THERMALLY-INDUCED RETRO-OXY-COPE REARRANGEMENT. Steven W. Elmorel and Leo A. Paquette
2.
How one might induce room temperature hydrolysis of a peptide

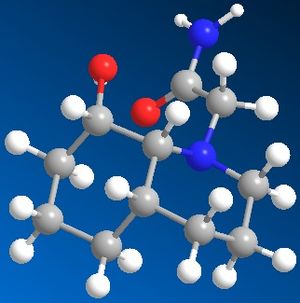
The first compound contains a cis-decalin ring, this can be in the chair-chair conformation or can ring flip into the chair-boat conformation, in this exercise only the chair-chair conformation is being analysed. The compound with the N-substituent equatorial to the cis-decalin ring had an overall energy of 16.35kcal/mol. The compound with the N-substituent axial to the cis-decalin ring had an overall energy of 19.78kcal/mol. This means the N-substituent in the equatorial position is more stable than in the axial position.
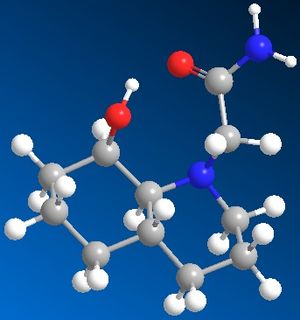

The secound compound contains a trans-decalin ring, this can only be in the chair-chair conformation and can't ring flip. The compound with the N-subtituent axial to the trans-decalin ring had an overall energy of 12.54kcal/mol. The compound with the N-substiuent equatorial to the trans-decalin ring had an overall energy of 14.10kcal/mol. This means that the compound is more stable with the N-substituent in the axial position.
These stabilities most likely rise from hydrogen bonding between the carbonyl and the alcohol. It is clearly visible from the image of the trans-decalin ring isomers that in the axial isomer, the carbonyl is clealy close enough to the alcohol to fom a hydrogen bond and stabilise the compound. The same goes for the cis-decalin ring isomers except in this case it is the equatorial isomer that is stabilised by the hydrogen bonding.

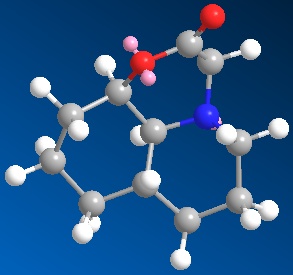
The product from hydrolysis of the cis-decalin compound results in a compound with energy of 35.79kcal/mol, the substiutents both being in the equatorial position off of the decalin ring. The product from hydrolysis of the trans-decalin compound results in a compound with energy 30.41kcal/mol, the substituents both axial off of the decalin ring. This shows that the reaction is thermodynamically controlled a both products show the same stereochemistry as the most stable isomer of the reactant.
The peptides hydrolyse much faster than other peptides do because it is an intramolecular reaction. An intermolecular reaction would require two components to collide in space with enough energy to react successfully, whereas the intramolcular reaction doesn't require the collisions so has more chance of the reaction proceeding and hence a faster reaction. The alcohol and the carbonyl group are also very close in both compounds, which aids in speeding up the reaction as there is a greater chance of the groups interacting. The cis-decalin ring reacts slightly faster as the substituents are closer together than the trans-decalin ring.
References
1. Rapid Cleavage of Unactivated, Unstrained Amide Bonds at Neutral pH. Nicolette M. Fernandes, Fabienne Fache, Mari Rosen, Phuong-Lan Nguyen, and David E. Hansen.
Regioselective Addition of Dichlorocarbene
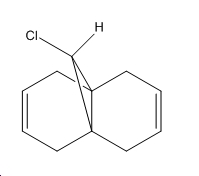
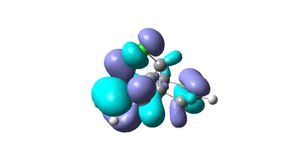
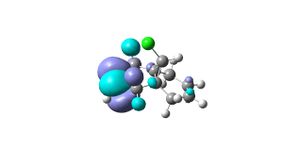
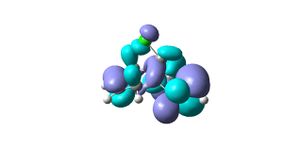
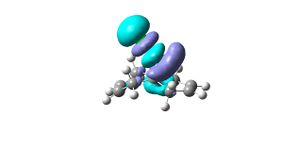
From the results returned from the DFT, the IR frequencies for molecule 12 have been interpreted as:
Frequency: Functional Group:
690.42 C-Cl bend
772.63 C-Cl stretch
1368.94 CH bend, alkane
3001.59 C-H stretch, alkane
3011.78 C-H stretch, alkene
3033.42 C-H stretch, alkene
3178.05 C-H stretch, alkene

From the results returned from the DFT, the IR frequencies for molecule 13 have been interpreted as:
Frequency: Functional Group:
682.74 C-Cl
782.37 C-Cl
943.90 =C-H bend
1328.44 C-H bend, alkane
3009.18 C-H stretch, alkane
3015.43 C-H stretch, alkene
3031.86 C-H stretch, alkene
3056.6 C-H stretch, alkene
3098.46 C-H stretch, alkene
3176.69 C-H stretch, alkene
C=C bonds should show stretches around 1640-1680cm-1, (1400-1500 and 1585-1600cm-1 if its an aromatic). There are no strong vibrations for these frequencies in the data from the modelling programme. The exo bond should have a higher frequency than the endo because it is more stable so should be stronger and therefore require more energy for vibration.
The double bond anti (endo) to the chloro group is hydrogenated easier than the other double bond (exo). The interactions between C-Cl σ* and the exo double bond stabilse the exo bond, and leave the endo bond more susceptible to attack by an electrophile. These interactions distort the molecule by bending the bridgehead carbon towards the exo double bond, this results in the overlap of the LUMO+2(C-Cl) and HOMO-1(exo bond) which stabilises the exo bond compared to the endo(HOMO). The HOMO-1 is more delocalised than the HOMO, so the pi-orbital contributions for the endo carbons are higher than those for the exo bond. The endo bond is also more sterically accessible than the exo bond.
References
1. A Molecular Orbital and Crystallographic Study of the Structure and pi-Facial Regioselectivity of 9-Chloro-1,4,5,8-tetrahydro-4a,8a-methanonaphthalene. Brian Halton, Roland Boeseb and Henry S. Rzepa
2. http://academic.pgcc.edu/~ssinex/IR_spect.ppt
Mini Project
Assigning Regioisomers in "Click Chemistry"
The products from the 1,3 cycloaddition between an azide and an alkyne are the two regioisomers of a 1,2,3-triazole. A is the 1,5-isomer and B is the 1,4-somer. When the reaction is catalysed by Cu(I), product B is favoured but when a Ru(II) catalyst is used, product A is favoured. C13 NMR can be used to tell the two isomers apart as the Carbons are in different environments so will show different chemical shits. H NMR won't show much difference as the hydrogens will be in almost the same environments. IR won't show much difference between the isomers as both isomers have the same functional groups.
Molecule A
Molecule A |
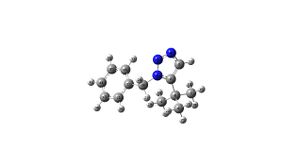
C13 NMR Results: DOI:10042/to-994
C8 = 29ppm
C9 = 29.7ppm
These values are very close together so may be seen together.
C7 = 31.6ppm
C6 = 32ppm
These values are also very close together so may be seen as a single peak.
C10 = 54.3ppm
C16 = 123ppm
C12 = 123.3ppm
These carbons are in similar positions so will probably show one peak.
C14 = 124.5ppm
C13 = 125.3ppm
C15 = 125.9ppm
These carbons are also in similar positions so will probably show one peak.
C3 = 128.7ppm
C11 = 134.3ppm
C2 = 142ppm
Molecule B
DOI:10042/to-995
Molecule B |
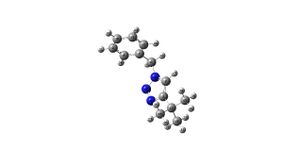
C13 NMR Results:
C15 = 28.1ppm
C16 = 31.2ppm
C14 = 31.5ppm
C13 = 34.9ppm
C6 = 54.4ppm
C2 = 115.6ppm
C9, C10, C11 = 124.9ppm
C12 = 125.6ppm
C8 = 126.7ppm
C7 = 132.8ppm
C3 = 152.7ppm
From Literature:
13C NMR (CDCl3): δ 29.72, 29.99, 52.60, 126.32, 127.68, 128.61, 131.26, 131.37, 136.37, 146.00.
These values show that molecule A corresponds to the data from the literature.
The mechanism for reaction proceeds via the following intermediates:
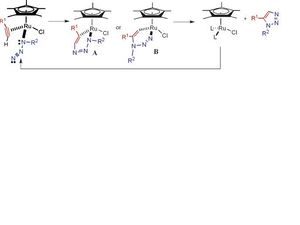
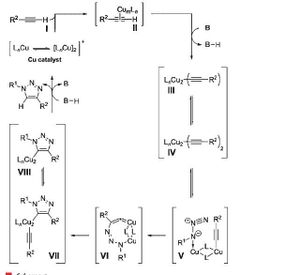
This shows that whether the 1,4-isomer or 1,5-isomer is produced depends on how the azide co-ordinates as a ligand to the catalyst. If the azide co-ordinates to the metal centre via the free nitrogen terminal, the 1,5-isomer will be produced as in the next step where the azide and alkyne interact, the R groups will end up next to each other. If the azide co-ordinates to the metal centre via the R-group nitrogen terminal, the 1,4-isomer will be produced as the R groups are futher away from each other. Below is an illustration for the mechanism for the formation of B. It involves the azide co-ordinating to the copper catalyst at the R group nitrogen terminal. The mechanism for the formation of A would be similar but the azide would co-ordinate to the Ru catalyst at the free nitrogen terminal.
References
1. Ruthenium-catalysed cycloaddition of alkyne and organic azides, JACS (plus supporting information)
2. Click Chemistry: Copper Clusters Catalyse the Cycloaddition of Azides with Terminal Alkynes
3. ‘Click’ Chemistry in Polymer and Material Science: An Update
4. ‘Click’ Chemistry in Polymer and Materials Science
5. http://www.chemistry.msu.edu/courses/CEM958/FS04_SS05%5Cmolengraft.pdf
