Rep:Mod:rjg286
The Hydrogentation of Cyclopentadiene Dimer
Cyclopentadiene readily undergoes dimerisation through a Diels-Alder reaction (a type of pericyclic reaction) to form an adduct dimer[1]. Two possible stereoisomeric products can form as a result of dimerisation, either the exo or the endo dimer depending on the stereochemistry of the hydrogen atoms at the centre of the dimer, that have to be syn to each other (due to the nature of the Diels-Alder pericyclic reaction).
The different isomers are formed under different conditions depending on whether the dimerisation is initiated by either heat (thermal conditions) or by light activation. By considering the circumstances where dimerisation is caused by heat it is possible to model and analyse each isomer to determine if the endo or exo dimer is preferred (via considering molecular mechanics and using the MM2 method in ChemBio3D).
- Results and Analysis
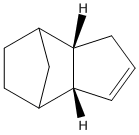
The numerical results for minimising the energy of both the exo and endo dimer are displayed in the tables below.
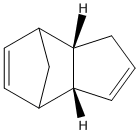
| Parameter | Energy (kcal/mol) |
|---|---|
| Stretch | 1.25 |
| Bend | 20.8 |
| Torsion | 9.51 |
| Van der Waals | 4.32 |
| Dipole | 0.448 |
| Total Energy | 34.0 |
| Parameter | Energy (kcal/mol) |
|---|---|
| Stretch | 1.29 |
| Bend | 20.5 |
| Torsion | 7.66 |
| Van der Waals | 4.23 |
| Dipole | 0.378 |
| Total Energy | 31.9 |
These results suggest that the endo dimer is the preferred isomer that results from dimerisation as it has a lower overall total energy than that of the exo dimer. As these isomers have a similar general structure the majority of each of the Molecular Modelling parameters are the same (or very similar). The main diffence in energy between the two isomers can be contributed to the greater torsional strain that is present in the exo dimer. This torsional strain in the exo dimer arises from the two 5-membered cycles of the dimer twisting towards each other, while in the endo dimer these cycles are twisting away from each other . This result has previously been confirmed[2]. Since the most stable isomer is formed this suggests that the dimerisation of cyclopentadiene is thermodynamically controlled.
- Hydrogenation of the Cyclopentadiene (Endo) Dimer
The hydrogenation of the endo cyclopentadiene dimer can occur at two different sites as there are two alkene bonds present in the dimer. Hydrogenation of the 5-membered cycloalkene ring give the hydrogenation product 3 while the hydrogenation of the 6-membered cycolalkene ring gives the hydrogentation product 4. The same MM2 method using ChemBio3D has been used to analyse the stability of the hydrogenation products 3 and 4.
- Results and Analysis
The numerical results for minimising the energy of both hydrogenation products 3 and 4 are displayed in the tables below.

| Parameter | Energy (kcal/mol) |
|---|---|
| Stretch | 1.25 |
| Bend | 19.2 |
| Torsion | 11.1 |
| Van der Waals | 5.80 |
| Dipole | 0.162 |
| Total Energy | 34.9 |
| Parameter | Energy (kcal/mol) |
|---|---|
| Stretch | 1.10 |
| Bend | 14.5 |
| Torsion | 12.5 |
| Van der Waals | 4.51 |
| Dipole | 0.141 |
| Total Energy | 31.2 |
These results suggest that product 4 is the more thermodynamically stable than product 3 as product 4 has a total lower energy than product 3. The difference in energy between the two products is roughly 4 kcal/mol most of which can be attritubted to a difference in bending energy. The bending energy of product 4 is less than that of product 3 because the creation of a 6-membered cycloalkane ring provides the 'optimal' geometry for a ring strucutre in product 4 which is not created in product 3. The rest of the molecular modelling parameters are very similar between the two products.
If the hydrogenation reaction is thermodynamically controlled product 4 is expected to be the major isomer that is formed. However once a hydrogen molecule been added across an alkene bond it cannot dissociate and reform H2. Therefore the hydrogenation reaction is not reversible so kinetics will control the addition of H2 to the dimer. From this analysis product 3 is expected to be the major product formed as the alkene bond in the 5-membered ring is less sterically hindered compared to the alkene in the 6-membered ring which is partially shielded by the bridging methlyene group.
An Investigating into the Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
An intermediate in the synthesis of taxol, a cancer treatment drug, either has its carbonyl group pointing upwards (9) or downards (10). Upon standing the intermediate isomerises so the carbonyl group is pointing in the opposite direction. Although these two isomers are similar in many respects they are sufficiently different to be considered as different molecules, atropisomers[1]. (These atropisomers are displayed in the Jmol links below) The only difference between the two isomers is a rotation of about a σ bond. However in these isomers this rotation is not trivial (unlike many organic compounds) due the sterical demands of substituent groups.
Intermediate in the synthesis of taxol, with the carbonyl group pointing up (9).
Intermediate in the synthesis of taxol, with the carbonyl group pointing down (10).
In order to dertmine which of these is most stable the same modelling method (MM2) in ChemBio3D will be used to minimise their energies.
Results and Analysis
The numerical results for minimising the energy of both antroisomeric forms 9 and 10 are displayed in the tables below.
| Parameter | Energy (kc/mol) |
|---|---|
| Stretch | 6.67 |
| Bend | 87.2 |
| Torsion | 19.6 |
| Van der Waals | 30.1 |
| Dipole | -2.89 |
| Total Energy | 142 |
| Parameter | Energy (kc/mol) |
|---|---|
| Stretch | 2.91 |
| Bend | 16.8 |
| Torsion | 20.2 |
| Van der Waals | 14.3 |
| Dipole | -1.72 |
| Total Energy | 53.2 |
These results suggest that isomer that has the carbonyl group point down (10) is the more stable isomer as it has a lower total energy than the isomer with the carbonyl pointing in the up direction (9). There is relatively large difference between the total energies of the isomers (around 90 kc/mol) the majority of which can be attributed to extra bending energy that is present in isomer 9. This extra bending energy arises from the 'twist boat' geometry that the 6-membered ring has to adopt in this atropisomer while the more stable isomer 10 has the more favourable lower energy chair geometry[2]. There is also an unfavourable transannular interaction between the carbonyl oxygen and the alkene proton when the carbonyl group is pointing up in isomer 9.
The alkene bond present in these isomers is 'hyperstable' meaning that it reacts at a much slower rate than normal alkene bonds. These bridgehead alkenes react slower than normal alkenes because of out of plane deformation of the overlapping p orbitals of the alkene bond, so that there is less overlap between them[3] (the creation of an angle dependency). This is forced onto the alkene bond due to the nature of the bridghead. This deformation makes the antibonding π* orbital harder to attack and therefore these alkene bonds are more stable.
Through using the MMFF94 method (in ChemBio3D) to minimise the energy of both isomers it was possible to confirm that isomer 10with the carbonyl group pointing down as the most stable isomer as it gave a similar magnitude of the total energies for both isomers.
Regioselective Addition of Dichlorocarbene
Modelling using molecular mechanics is limited because it does not consider electron interactions with a molecle which are extremely important as these interactions control the reactivity and spectroscopic properties of all molecules. During this exercise electronic interactions will be taken into account.
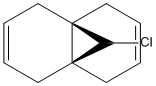
Compound 12 is unstaurated and will react with electrophilic reagents such as a dichlorocarbene. The regiochemistry of this molecule is carefully controlled by the presence of the chlorine atom. The effect of the presence of this chlorine atom on the regiochemistry (whether electrophilic addition occurs at the π bond that is closer or further from the chlorine atom)
will be investigated by considering the molecular oritbal surfaces of 12.
- Molecular Orbital Surfaces
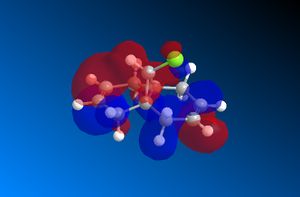


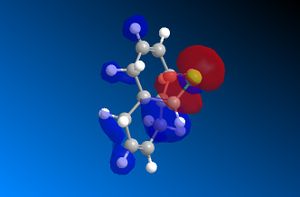
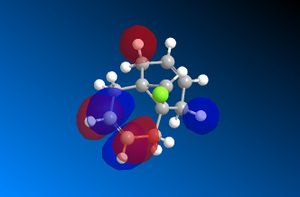
The important molecular orbitals surfaces of compound 12 have been calculated using the MOPAC/PM6 method in ChemBio3D. These orbitals are displayed below. These molecular orbitals surfaces show the distribution of the electron density across the molecule for each molecular orbital.
The HOMO-1 molecular orbital, which is occupied by electrons in the ground state of compound 12, shows strong bonding interactions across the molecule. The positive (red surface) phase electron density occupies the top face of the 6-membered ring on the opposite side of the chlorine atom (shown in green), while the negative (blue surface) phase electron density occupies the underface of the molecule. On the side with the chlorine atom the phases of the p like oritbal, localised on the chlorine atom, interacts strongly with π bond that it is close to. Overall this creates bonding interactions in the HOMO-1.
The HOMO molecule orbital, the highest energy molecular orbital that is occupied, is key to explaining the reactivity of 12 where it acts as an electron donor because the electrons involved in this reaction will come from the HOMO. This HOMO shows that there is a significant difference in the electron distribution between the two alkene bonds. The alkene bond with the largest electron density (denoted by the size of the surface) localised on it in this HOMO is the alkene bond that is closest to the chlorine atom. Therefore it is expected that this alkene bond will undergo additon before the alkene bond which is further away from the chlorine atom because this further alkene bond is less electron rich than the nearer alkene bond in the HOMO of compound 12. This has previously been reported[1].
The LUMO of 12, which is not occupied by electrons, is dominated by the distinctive antibonding character of the п* orbital associated with the alkene bond that is furthest away from the chlorine atom. There is some slight delocalisation between this п* orbital and the hydrogen on the bridging carbon atom.
The LUMO+1 of 12 is dominated by the Cl-C σ* bond. This Cl-C σ* bond is thought to overlap with the п bond that is furthest from the chlorine atom which results in a stabilising interaction1. This creates an energy difference between the two alkene bonds in 12 which creates the regiochemistry that controls the electrophilic addition to 12.
The LUMO+2 is very symmetric and consists mainly of the п* orbitals from the alkene bond nearest to the chlorine atom (i.e. not the alkene bond in the LUMO).
This exercise has demonstrated the importance of considering molecular orbital electronic interactions as they can dominate the reactivity of a particular functional group within a molecule.
- ↑ B. Halton, R. Boese and H.S. Rzepa, J. Chem. Soc. Perkin Trans. 2, 1992, p447 - 450
- Electronic Influence on Spectroscopic Properties

The electronic strcutre of a molecule not only dominates its reactivity but also its spectroscopic properties. This will be investigated by considering the effect of the presence of the chlorine atom on the vibrations of compound 12 and its monohydrogenated derivative 13. This will be done by using the density functional approach to obtain the calculated vibrational spectra of these molecules.
Molecule 12 displays a Cl-C stretch at 771 cm-1 and two C=C stretching frequencies, one at 1737cm-1 relating to the C=C bond furthest from the chlorine atom and the other at 1757cm-1 which corresponds to the C=C stretch of the alkene bond cloest to the chlorine atom. These vibrations can be seen below[1].
Animation showing the Cl-C stretch in 12 at 771cm-1.
Animation showing the C=C stretch of the exo C=C bond (furthest C=C bond to the chlorine atom) in 12 at 1737cm-1.
Animation showing the C=C stretch of the endo C=C bond (nearest C=C bond to the chlorine atom) in 12 at 1757cm-1.
Molecule 13 displays a Cl-C stretch at 775 cm-1 and one C=C stretching frequency at 1758cm-1 relating to the endo C=C bond stretch. These vibrations can be seen below[2].
Animation showing the Cl-C stretch in 13 at 775cm-1.
Animation showing the C=C stretch of the endo C=C bond in 13 at 1755cm-1.
This vibrational information shows that Cl-C stretch at 771cm-1 in 12 has an influence on the exo C=C as this C=C bond also shows movement when the Cl-C bond stretches. This influence is what causes the two π bonds to have different vibrational frequencies. The electronic distribution of the Cl-C σ* bond is perfectly aligned to interact with exo C=C. This results in partial delocalisation of the electrons in the exo π bond into the σ* Cl-C which is turn reduces the bond order between the two carbon atoms in the exo C=C bond. So this bond is weaker and therefore stretches at a lower frequency (1737cm-1 compared to 1757 cm-1 of the endo C=C bond).
This effect is also reflected in the stretching frequencies of the Cl-C bond in 12 and 13. Occupation of the σ* Cl-C bond, in 12, weakens the Cl-C σ bond to stretch at 771-1 compared to 775cm-1 in 13 where the occupation of the σ* Cl-C bond does not occur as there is no exo C=C bond to donate electron into it.
This result shows that the density functional approach is a simple and efficient method to calculate and compare vibrations in different molecules. Analysis of the vibrations calculated by this method can only be appreicated by examining the key electronic interactions within the molecule investigated.