Rep:Mod:ri3717
NH3 molecule
Summary Information
N-H bond distance = 1.01779 Å
H-N-H bond angle = 105.757°
Molecule name: NH3
Calculation method: RB3LYP
Basis set: 6-31G(d,p)
Final energy E(RB3LYP): -56.55776866 a.u.
Point group: C3V
Convergence
Item table:
Item Value Threshold Converged? Maximum Force 0.000137 0.000450 YES RMS Force 0.000090 0.000300 YES Maximum Displacement 0.000352 0.001800 YES RMS Displacement 0.000189 0.001200 YES
NH3 |
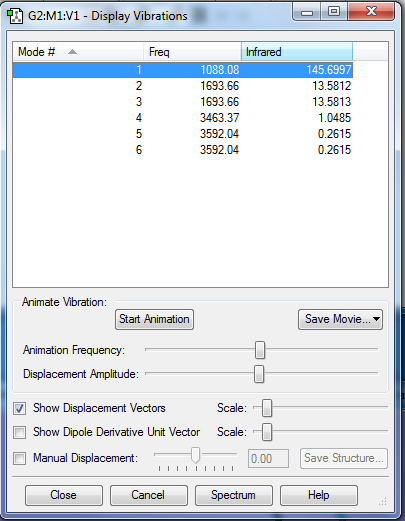
Vibration frequencies
Questions:
1. How many modes do you expect from the 3N-6 rule?
6 modes
2. Which modes are degenerate (i.e. have the same energy)?
2 and 3 are degenerate.
5 and 6 are degenerate.
3. Which modes are "bending" vibrations and which are "bond stretch" vibrations?
1, 2 and 3 are bending, 4, 5, and 6 are stretching.
4. Which mode is highly symmetric?
Mode 4, and 5 and 6
5. One mode is known as the "umbrella" mode, which one is this?
Mode 1
6. How many bands would you expect to see in an experimental spectrum of gaseous ammonia?
2 bands
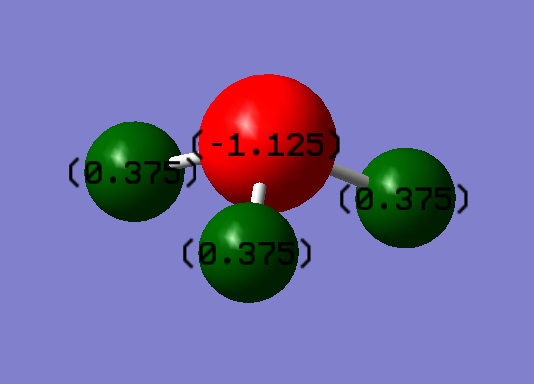
Atomic charges
Atomic charges: -1.125 on N, +0.375 on Hs. This agrees with expectation because the electronegativity of nitrogen is greater than hydrogen, so there should be a larger electron cloud around nitrogen.
Independent work: Comparison against literature values
Literature value
N-H bond distance = 1.0124 Å [1]
H-N-H bond angle = 106.670° [1]
Computer generated values
N-H bond distance = 1.01779 Å
Error = (1.01779-1.0124)/1.0124 = 0.005323982... = 0.5% (1 s.f.)
H-N-H bond angle = 105.757°
Error = (105.757-106.670)/106.670 = -0.008559107...
Taking the absolute value of this gives:
Error = 0.9% (1 s.f.)
N2 molecule
Summary information
N-N bond distance = 1.10550 Å
Molecule name: N2
Calculation method: RB3LYP
Basis set: 6-31G(d,p)
Final energy E(RB3LYP): -109.52412868 a.u.
Point group: D*H
Convergence
Item table:
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
N2 |
Vibration frequencies
Atomic charges
N2 consists of two nitrogen atoms, and there is no difference in electronegativity. Therefore, the N-N bond in the nitrogen molecule is 100% covalent and there are no charges.
H2 molecule
Summary information
H-H bond distance = 0.74279 Å
Molecule name: H2
Calculation method: RB3LYP
Basis set: 6-31G(d,p)
Final energy E(RB3LYP): -1.17853936 a.u.
Point group: D*H
Convergence
Item table:
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
H2 |
Vibration frequencies
Atomic charges
H2 consists of two hydrogen atoms, and there is no difference in electronegativity. Therefore, the H-H bond in the hydrogen molecule is 100% covalent and there are no charges.
Energy calculation
E(NH3)= -56.55776866 a.u.
2*E(NH3)= -113.11553732 a.u.
E(N2)= -109.52412868 a.u.
E(H2)= -1.17853936 a.u.
3*E(H2)= -3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579062 a.u.
ΔE= -146.4782... = -146.49 kJ/mol
Since the energy change is negative, the ammonia product is more energetically stable than the gaseous reactants.
Project molecule: O2
Summary Information
O-O bond distance = 1.21602 Å
Molecule name: O2
Calculation method: RB3LYP
Basis set: 6-31G(d,p)
Final energy E(RB3LYP): -150.25742434 a.u.
Point group: D*H
Convergence
Item table:
Item Value Threshold Converged? Maximum Force 0.000130 0.000450 YES RMS Force 0.000130 0.000300 YES Maximum Displacement 0.000080 0.001800 YES RMS Displacement 0.000113 0.001200 YES
O2 |
Vibration frequencies
Atomic charges
O2 consists of two oxygen atoms, and there is no difference in electronegativity. Therefore, the O-O bond in the oxygen molecule is 100% covalent and there are no charges.
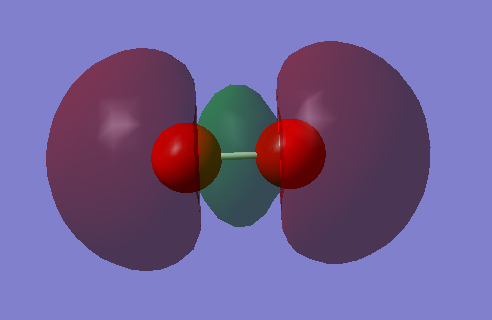
Molecular orbitals
MO1
Two s orbitals from oxygen atoms contribute to this molecular orbital. This is an occupied bonding orbital that is lower in energy than the original s orbitals.
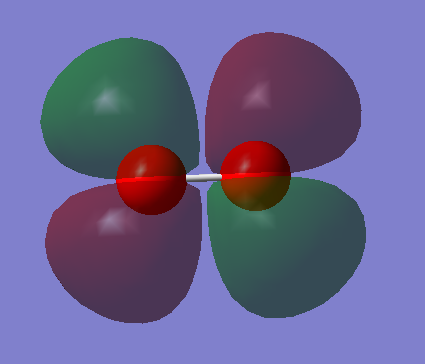
MO2
Two s orbitals from oxygen atoms contribute to this molecular orbital. This is an occupied anti-bonding orbital that is higher in energy than the original s orbitals.
MO3
This is an occupied anti-bonding orbital that is formed by the axial overlap of two p orbitals and the resultant molecular orbital is higher in energy than the original p orbitals.
MO4
This is an occupied bonding orbital that is formed by the parallel overlap of the two p orbitals and the resultant molecular orbital is lower in energy than the original p orbitals.
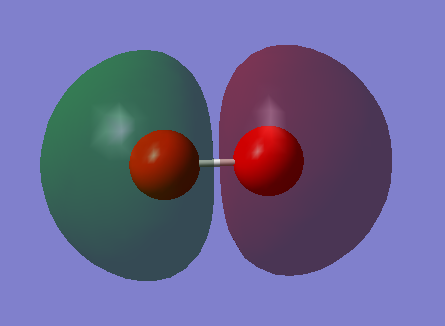
MO5
This is an unoccupied anti-bonding orbital that is formed by the parallel overlap of the two p orbitals and the resultant molecular orbital is lower in energy than the original p orbitals. This is the LUMO of O2.
References
- ↑ 1.0 1.1 National Institute of Standards and Technology. (2016) Experimental data for NH3. Available from https://cccbdb.nist.gov/exp2x.asp?casno=7664417 [Accessed 1 March 2018]