Rep:Mod:qx5117
EX3 Section
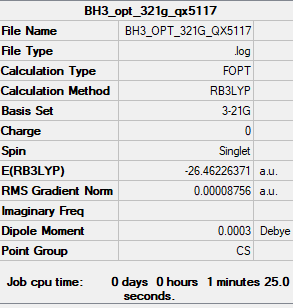
BH3
B3LYP/3-21G level
Item Value Threshold Converged? Maximum Force 0.000217 0.000450 YES RMS Force 0.000105 0.000300 YES Maximum Displacement 0.000900 0.001800 YES RMS Displacement 0.000441 0.001200 YES
This calculation isn't necessarily relevant to the report and could have done with a supporting comment of maybe why the calculation was run? etc. Smf115 (talk) 07:20, 30 May 2019 (BST)
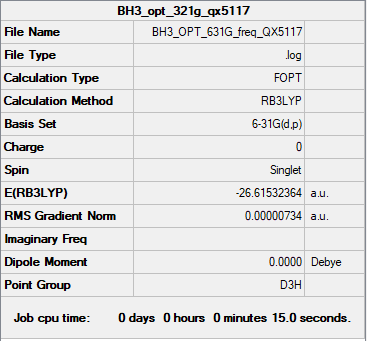
B3LYP/6-31G level
E = -26.61532 a.u.
Item Value Threshold Converged? Maximum Force 0.000012 0.000450 YES RMS Force 0.000008 0.000300 YES Maximum Displacement 0.000064 0.001800 YES RMS Displacement 0.000039 0.001200 YES
Low frequencies --- -10.3498 -3.4492 -1.2454 -0.0054 0.4779 3.2165 Low frequencies --- 1162.9519 1213.1527 1213.1554
BH3 molecule |
frequency analysis
Frequency analysis log file: Media:BH3 OPT 631G FREQ QX5117.LOG
| wavenumber (cm-1 ) | Intensity (arbitrary units) | symmetry | IR active? |
type |
|---|---|---|---|---|
| 1163 | 93 | A2 | yes | out-of-plane bend |
| 1213 | 14 | E | yes | bend |
| 1213 | 14 | E | yes | bend |
| 2582 | 0 | A1 | no | symmetric stretch |
| 2716 | 126 | E | yes | asymmetric stretch |
| 2716 | 126 | E | yes | asymmetric stretch |
Q:Why are there less than six peaks in the spectrum, when there are obviously six vibrations?
Because there is a symmetric stretch at 2582 cm-1 with zero intensity, so no peak. And there are two sets of identical vibration at 1213 and 2716 cm-1. For these four vibration, there are only two peaks observed as these are degenerate. In total, there are only three peaks observed at 1163, 1213, 2716 cm-1.
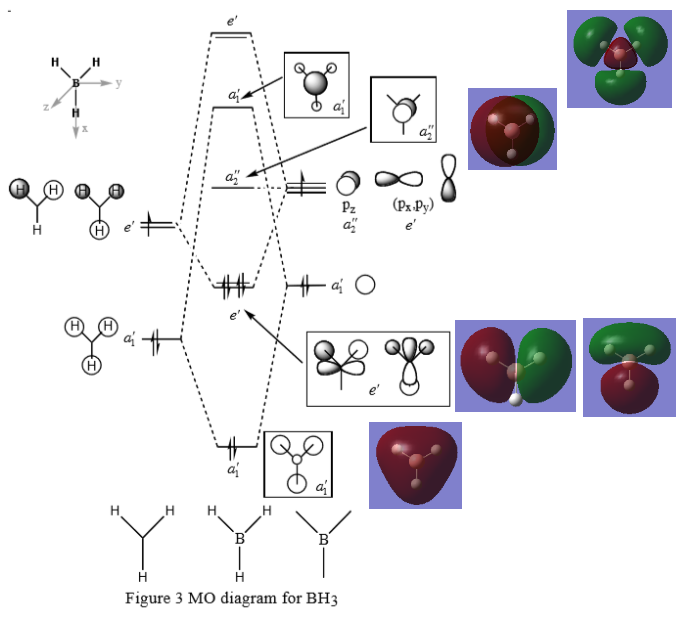
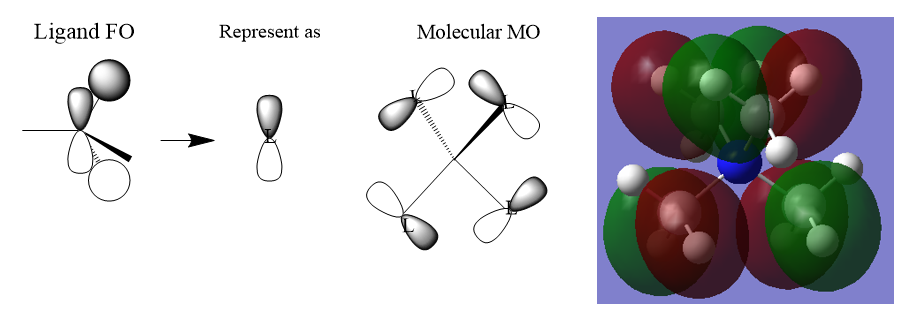
MO of BH3
- ↑ Hunt research group. 2019. Lecture_4_Tut_MO_diagram_BH3. [ONLINE] Available at: http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf. [Accessed 17 May 2019].
Q:Are there any significant differences between the real and LCAO MOs?
The LCAO can be used to predict the molecular orbitals and it shows the additive of two orbitals directly. The real MOs from optimisation shows how the orbitals look like after the orbitals are added up and mix in the LCAO. In the real MOs, the in phase part of orbitals will mix up well to become a cloud and the out of phase part will have repulsion between each other so the direction of the MOs after mixing will shift from the original position.
Q:What does this say about the accuracy and usefulness of qualitative MO theory?
The LCAO only allows us to predict the MOs by addition of the orbitals directly. It can be only used to produce a qualitative MO diagram and to compare the relative energy of different orbitals. Calculation is required to obtain the real energy of the MOs. By mixing the LCAO, we can also obtain the similar shapes of the real MOs but only in a qualitative way not the precise size of the real MOs.
Clear inclusion of some of the calculated MOs, however, you are missing both the LCAO and calculates MOs for the top two e' MOs. Good consideration of the differences between the method, which could be improved by considering one of the actual MOs from the diagram (e.g. 3a1) and could be clearer in the language used. Smf115 (talk) 07:14, 30 May 2019 (BST)
NH3 + BH3 reaction
E(NH3) = -56.55777 a.u. E(BH3) = -26.61532 a.u. E(NH3BH3) = -83.22469 a.u.
ΔE= -0.0516 a.u. = -135 kJ/mol
Correct final answer but there is no evidence of the calculation and you are missing the supporting structures and corresponding information for NH3 and NH3BH3. Smf115 (talk) 07:17, 30 May 2019 (BST)
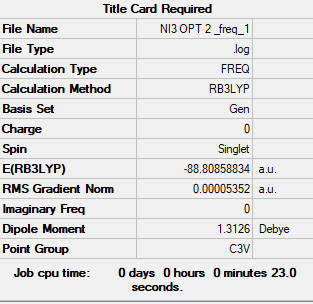
NI3
B3LYP/6-31G(d,p)LANL2DZ NI3
Summary table
Item Value Threshold Converged? Maximum Force 0.000344 0.000450 YES RMS Force 0.000213 0.000300 YES Maximum Displacement 0.000831 0.001800 YES RMS Displacement 0.000486 0.001200 YES
Frequency analysis log file: Media:NI3 OPT 2 FREQ 1.LOG
Low frequencies --- -12.6866 -12.6806 -6.4059 -0.0039 0.0190 0.0622 Low frequencies --- 101.0816 101.0824 147.4566
NI3 molecule |
N-I bond distance = 2.18 Å
Correct implementation of the pseudopotential (PP) and nice presentation of the full method and PP used at the start of the section. Overall the missing structure and a few mistake let the first section down. Smf115 (talk) 07:21, 30 May 2019 (BST)
mini project Section
B3LYP/6-31G level
[N(CH3)4]+
Item Value Threshold Converged? Maximum Force 0.000124 0.000450 YES RMS Force 0.000065 0.000300 YES Maximum Displacement 0.000868 0.001800 YES RMS Displacement 0.000440 0.001200 YES
Frequency analysis log file: Media:NCH4 OPT 1 FREQ 1.LOG
Low frequencies --- -0.0013 -0.0009 -0.0007 35.2749 35.2749 35.2749 Low frequencies --- 218.6733 317.3870 317.3870
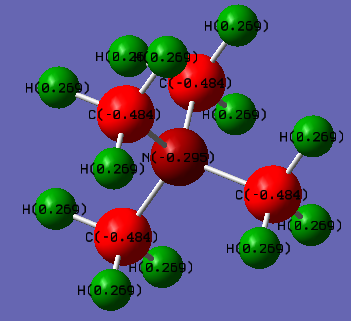
[N(CH3)4]+ molecule |
N-C bond = 1.51 Å C-H bond = 1.09 Å
[P(CH3)4]+
Item Value Threshold Converged? Maximum Force 0.000112 0.000450 YES RMS Force 0.000033 0.000300 YES Maximum Displacement 0.000938 0.001800 YES RMS Displacement 0.000506 0.001200 YES
Frequency analysis log file: Media:PCH4 OPT 1 FREQ 3.LOG
Low frequencies --- -0.0023 -0.0020 -0.0014 23.0634 23.0634 23.0634 Low frequencies --- 160.4414 195.1775 195.1775
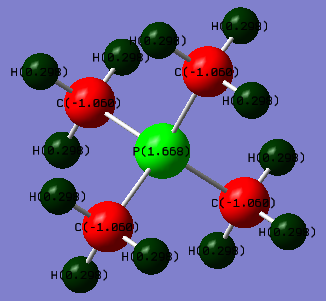
[P(CH3)4]+ molecule |
P-C bond = 1.82 Å C-H bond = 1.09 Å
Compare the charge distribution for these cations
[N(CH3)4]+ charge distribution
[P(CH3)4]+ charge distribution
| Molecules | H | C | N/P |
|---|---|---|---|
| [N(CH3)4]+ | 0.27 | -0.48 | -0.30 |
| [P(CH3)4]+ | 0.30 | -1.06 | 1.67 |
From the data above, we can notice that in [N(CH3)4]+, N and Cs carries the negative charge and the hydrogen carries the positive charge. That is due to the different electronegativity of the atoms. From the periodic table, the electronegative are in order of C > N > H. Therefore, Cs is the most negative atoms in the cation which pull the electrons toward themselves and make the Hs become positive in the cation. N is also electronegative so also carries the negative charge on it. For [P(CH3)4]+, the electronegative is in order of C > P > H. The carbon pull electrons on Hs and P making the P and Hs become positive. Because C is higher in electronegativity.
Correct NBO charges calculated, however, the same colour range should have been used across both ILs to compare the charge distributions. You are correct in comparing the relative electronegativities to justify the charges but your analysis could have been clearer and should have also considered other effect such as symmetry.Smf115 (talk) 14:51, 30 May 2019 (BST)
What does the "formal" positive charge on the N represent in the traditional picture?
It means there is a dative bond between one of the N and H. The lone pair electrons on nitrogen contribute to form the covalent bond. So from this representation, the N is positive because loss of one of the hydrogen make it become positive.
On what atoms is the positive charge actually located for this cation?
The H atoms are carrying the positive charge from the calculation in Gaussian.
Correct observation that the H's carry the positive charge but you needed to consider formal electron counting when thinking about the traditional picture of the charge location. Smf115 (talk) 14:51, 30 May 2019 (BST)
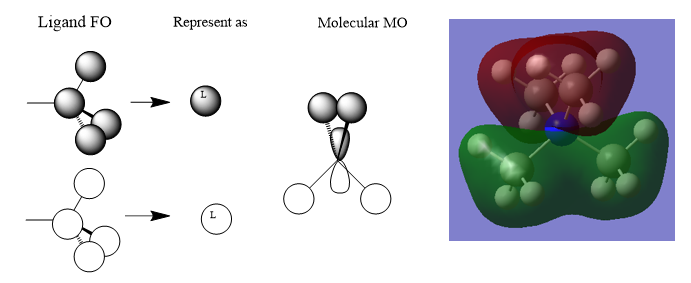
MOs of [N(CH3)4]+
Correct FOs and LCAOs for all three MOs which is good. However, you haven't labelled which MOs were selected and it would have been good to see some attempt at describing the main interactions and the character of the MOs. Smf115 (talk) 14:56, 30 May 2019 (BST)
Overall a decent report which lacks some analysis in the project section. Smf115 (talk) 14:56, 30 May 2019 (BST)