Rep:Mod:qwt11
Experiment 1C: Organic Computational Lab
The Hydrogenation of Cyclopentadiene Dimer
Kinetic control VS Thermodynamic control
exo |
Dimer 1
exo |
Dimer 2
exo |
Dimer 3
exo |
Dimer 4
Cyclopentadiene dimerises to an endo dimer and an exo dimer, 1&2. Hydrogenation of these two dimers proceeds to give two relative derivatives 3&4. The aim is to distinguish which product undergoes a thermodynamic or a kinetic reaction. A series of chemical related tools are used. “Chembio3D” shows both planar and stereo versions of molecules. "Gaussview" uses to display predicted NMR spectra. Under the help of “Avogadro”, a few types of crucial energy obtain, such as total bond stretching energy, total angle bending energy, total stretch bending energy, total torsional energy, total out-of-plane bending energy, total Van der Waals energy, total electrostatic energy and total energy (sum) of a particular. These information are allocated and the table below lists them out.
| Property | Dimer 1 (kcal/mol) | Dimer 2 (kcal/mol) | Dimer 3 (kcal/mol) | Dimer 4 (kcal/mol) |
|---|---|---|---|---|
| Total bond stretching energy | 3.54360 | 3.46789 | 3.31086 | 2.93009 |
| Total angle bending energy | 30.77335 | 33.18937 | 31.92713 | 21.09197 |
| Total stretch bending energy | -2.04136 | -2.08218 | -2.10106 | -1.70918 |
| Total torsional energy | -2.73744 | -2.94947 | -1.46254 | 0.17128 |
| Total out-of-plane bending energy | 0.01469 | 0.02183 | 0.01315 | 0.00051 |
| Total Van der Waals energy | 12.80707 | 12.35873 | 13.63924 | 10.68113 |
| Total electrostatic energy | 13.01379 | 14.18450 | 5.11948 | 5.14891 |
| Total energy | 55.37368 | 58.19067 | 50.44627 | 38.31470 |
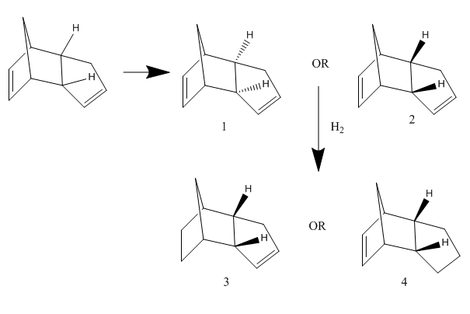
According to the table above, we clearly see that total energy (55.37368kcal/mol) of dimer 2 (55.37368kcal/mol) stays below while compare with dimer 1, which means dimer 1 is thermodynamic stable (ie: product stability) and dimer 2 is kinetic stable (ie: transition state stability). Likewise hydrogenated compound 3 gains more stability when it acts as a transition state and compound 4 prefers to act as a product. On the other hand, two indicated hydrogens senses more steric effect by the double bond in molecule 3, hence the higher total energy and less stable.

exo |
Intermediate9
exo |
Intermediate10
| Property | Intermediate 9 (kcal/mol) | Intermediate 10 (kcal/mol) |
|---|---|---|
| Total bond stretching energy | 7.70017 | 7.91408 |
| Total angle bending energy | 28.29773 | 20.97586 |
| Total stretch bending energy | -0.06950 | -0.06081 |
| Total torsional energy | 0.10769 | 4.45974 |
| Total out-of-plane bending energy | 0.98074 | 0.95594 |
| Total Van der Waals energy | 33.24245 | 34.75428 |
| Total electrostatic energy | 0.29497 | -0.04407 |
| Total energy | 70.55425 | 68.95502 |
Due to total energy shown on this table, we easily determine molecule 10 is more stable since it owns relatively lower total energy. On the other hand, we find that all the energy between two intermediates are virtually the same expect for the total angle bending energy. By using “Avogadro”, we prove that the angle between C-O-C(carbon on six-membered ring) for compound 10 is 108.5。 which exactly matches the angle of sp3 hybridization, however, the angle distorts to 117.5。 for compound 9 while a large gap indicates. Therefore, the final total energy of molecule 9 remains at a relatively high level.
Here is the proof:
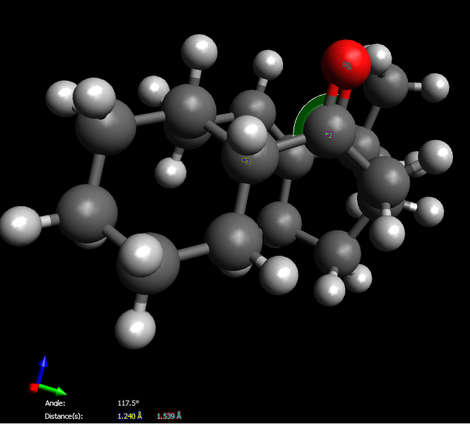
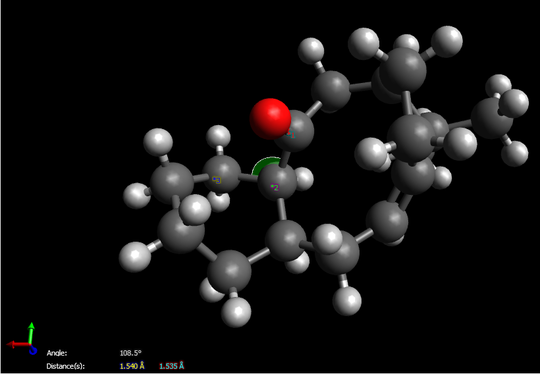
Futhermore, for the reason that why alkenes particular for 9 and 10 react slowly. A new class of “hyperstable” olefins can now be defined, olefins which contain less strain than that of the parent hydrocarbon and have negative OS values. Such olefins should be very unreactive-not due to steric hindrance3’ or to enhanced a-bond strength but due to special stability afforded by the cage structure of the olefin and to the greater strain of the parent polycycloalkane.[1]
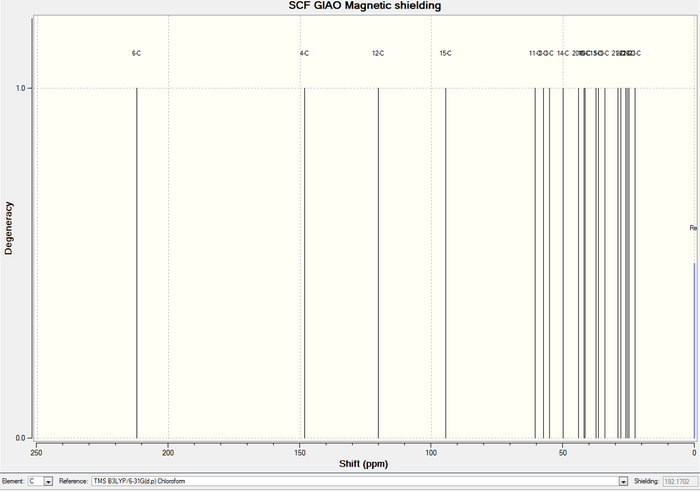
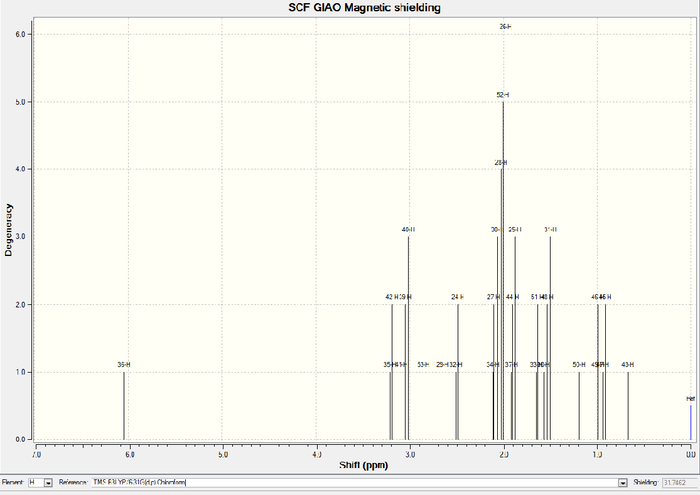

| chmeical shift (ppm) | Degeneracy | Atoms |
|---|---|---|
| 6.05 | 1 | 36 |
| 3.20 | 2 | 35,42 |
| 3.05 | 3 | 41,39,40 |
| 2.85 | 1 | 53 |
| 2.65 | 1 | 29 |
| 2.50 | 2 | 32,24 |
| 2.05 | 6 | 34,27,38,28,52,26 |
| 1.90 | 2 | 37,35 |
| 1.64 | 1 | 33 |
| 1.53 | 2 | 30,31 |
| 1.28 | 3 | 49 50 51 ave |
| 1.17 | 3 | 43 44 45 ave |
| 1.15 | 3 | 46 47 48 ave |
| chmeical shift (ppm) | Degeneracy | Coupling |
|---|---|---|
| 5.21 | 1 | multiplet |
| 3.00-2.70 | 6 | multiplet |
| 2.70-2.35 | 4 | multiplet |
| 2.20-1.70 | 6 | J=5.4HZ |
| 1.58 | 1 | multiplet |
| 1.50-1.20 | 3 | multiplet |
| 1.10 | 3 | singlet |
| 1.07 | 3 | singlet |
| 1.03 | 3 | singlet |
| chmeical shift (ppm) | Degeneracy | Atoms |
|---|---|---|
| 212.18 | 1 | 6 |
| 148.12 | 1 | 4 |
| 120.21 | 1 | 12 |
| 94.55 | 1 | 15 |
| 60.53 | 1 | 11 |
| 57.47 | 1 | 8 |
| 55.06 | 1 | 3 |
| 49.83 | 1 | 14 |
| 44.09 | 1 | 20 |
| 42.06 | 1 | 16 |
| 41.62 | 1 | 19 |
| 37.38 | 1 | 13 |
| 36.62 | 1 | 5 |
| 33.94 | 1 | 9 |
| 29.00 | 1 | 21 |
| 27.96 | 1 | 2 |
| 26.18 | 1 | 22 |
| 25.44 | 1 | 1 |
| 24.78 | 1 | 10 |
| 22.51 | 1 | 23 |
| chmeical shift (ppm) | Degeneracy | Atoms |
|---|---|---|
| 211.49 | 1 | 6 |
| 148.72 | 1 | 4 |
| 120.90 | 1 | 12 |
| 74.61 | 1 | 15 |
| 60.53 | 1 | 11 |
| 51.30 | 1 | 8 |
| 50.94 | 1 | 3 |
| 45.53 | 1 | 14 |
| 43.28 | 1 | 20 |
| 40.82 | 1 | 16 |
| 38.73 | 1 | 19 |
| 36.78 | 1 | 13 |
| 35.47 | 1 | 5 |
| 30.84 | 1 | 9 |
| 30.00 | 1 | 21 |
| 25.56 | 1 | 2 |
| 25.35 | 1 | 22 |
| 22.21 | 1 | 1 |
| 21.39 | 1 | 10 |
| 19.83 | 1 | 23 |
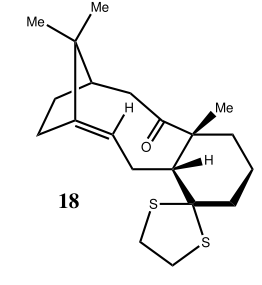
In 1H NMR of molecule 18, three easily distinguishable "ave" (average) list in table 1 since "Gaussview" always counts three Hs in methyl group as a singlet regardless of any other influences. Hence, find them out, average them and get the final result which is shown in table 1. In comparison with literature values of 1H NMR, main peaks almost match like degeneracy=6 and while chemical shift=1.58. Three methyl groups count a little distinction due to the systematic error for skip of adjacent electrogative atoms such as sulphur. There is about 1ppm deviation at carbonyl groups when compared with the referred values since two distinct solvents are used (CHCl3 and C6D6). Chloroform contains three very electronegative chlorine atoms to withdraw electron density and then leads to a more shielded carbonyl group.
Likewise, in table 3&4, only one exception appears. Carbon 15 in simulated spectrum directly connects with two sulphur atoms can be readily distorted since highly electronegative chlorine atoms in solvent contribute shielding effect in a huge extent. The site of sulphur attachment, carbon 15 is approximate 20 ppm too high (6-31G(d,p)/aug-cc-pVDZ bases, respectively). This is a well-known effect due to spin–orbit (SO) coupling and is exhibited increasingly by “heavy elements”. [3]

Thermal energy: The sum of electronic and thermal Energies= -1651.392841kJ/mol which stands by that the free energy is much less than zero. In another word, forward reaction will proceed spontaneous and the product is quite stable since a such a huge negative values gained.
