Rep:Mod:qwe107
NH3
Optimisation
Molecule- NH3
Calculation method- RB3LYP
Basis set- 6-31G(d.p)
Final energy E(RB3LYP) in atomic units (au)- -56.55776873
RMS gradient- 0.00000485
point group- C3V
Optimised bond length- 1.01798 nm
Optimised bond angle- 105.741°
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
The optimisation file is liked here
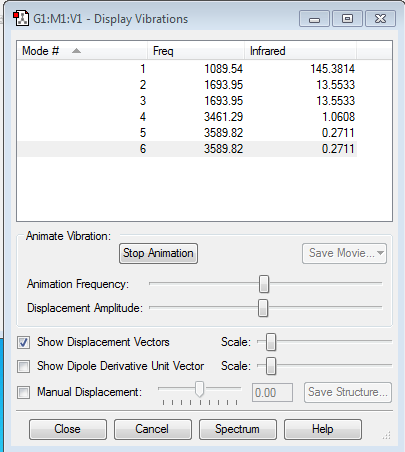
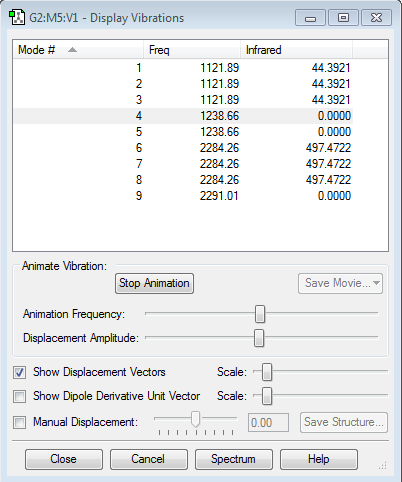
Frequency Analysis
Expected modes- (3X4)-6=6
Degenerate modes- modes 2 and 3 and modes 5 and 6
Bending vibrations- modes 1, 2, 3
Bond stretch vibrations- 4, 5, 6
Highly symmetric- mode 4
Umbrella mode- mode 1
Number of bands expected in an experimental spectrum- 3 however one has very flow intensity
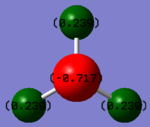
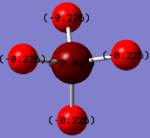
Charge Analysis
Charge on the N-atom- -1.125
Charge on the H-atom- +0.375
The expected charges are negative on the N-atom and positive on the H-atom as Nitrogen is more electronegative and so will attract a higher charge density.
N2
Optimisation
Molecule- N2
Calculation method- RB3LYP
Basis set- 6-31G(d.p)
Final energy E(RB3LYP) in atomic units (au)- -109.52412868
RMS gradient- 0.00000365
point group- D∞h
Optimised bond length- 1.10550 nm
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000002 0.001800 YES RMS Displacement 0.000003 0.001200 YES
The optimisation file is liked here
Frequency Analysis
There is no peaks on the IR spectrum as the vibration does not have a change in dipole moment.
H2
Optimisation
Molecule- H2
Calculation method- RB3LYP
Basis set- 6-31G(d.p)
Final energy E(RB3LYP) in atomic units (au)- -1.17853936
RMS gradient- 0.00002276
point group- D∞h
Optimised bond length- 0.74274 nm
Item Value Threshold Converged? Maximum Force 0.000039 0.000450 YES RMS Force 0.000039 0.000300 YES Maximum Displacement 0.000052 0.001800 YES RMS Displacement 0.000073 0.001200 YES
The optimisation file is liked here
Frequency Analysis
There is no peak on the IR spectrum as the vibration does not have a change in dipole moment.
Reaction energy for the formation of NH3
E(NH3)= -56.55776873 au
2*E(NH3)= -113.11553746 au
E(N2)= -109.52412868 au
E(H2)= -1.17853936 au
3*E(H2)= -3.53561808 au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579070 au
ΔE= -146.48 KJ/mol
Product, NH3, is more stable
[BH4]-
Optimisation
BH |
Molecule- BH4-
Calculation method- RB3LYP
Basis set- 6-31G(d.p)
Final energy E(RB3LYP) in atomic units (au)- -27.24992701
RMS gradient- 0.00000671
point group- TD
Optimised bond length- 1.23933 nm
Optimised bond angle- 109.471°
Item Value Threshold Converged? Maximum Force 0.000013 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000065 0.001800 YES RMS Displacement 0.000035 0.001200 YES
The optimisation file is liked here
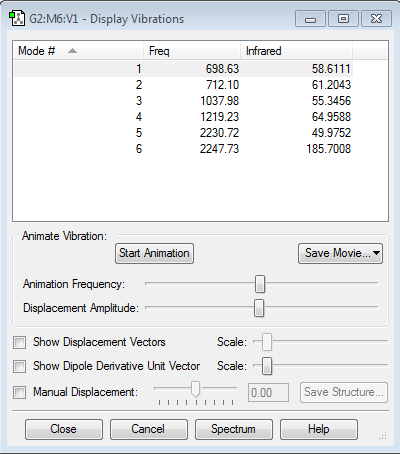
Frequency Analysis
Expected modes- (3X5)-6=9
Degenerate modes- modes 1, 2 and 3, modes 4 and 5 and modes 6, 7 and 8
Bending vibrations- modes 1 to 5
Bond stretch vibrations- 6 to 9
Symmetric- modes 4, 5 and 9
Number of bands expected in an experimental spectrum- 2
Charge Analysis
Charge on the B-atom- -0.097
Charge on the H-atom- -0.226
Molecular orbitals
H2SiO
Optimisation
Molecule- H2SiO
Calculation method- RB3LYP
Basis set- 6-31G(d.p)
Final energy E(RB3LYP) in atomic units (au)- -365.90001403
RMS gradient- 0.00000941
point group- CS
Optimised Si=O bond length- 1.53172
Optimised O-Si-H bond angle- 124.158°
Optimised Si-H bond length- 1.48652
Optimised H-Si-H bond angle- 111.686°
Item Value Threshold Converged? Maximum Force 0.000023 0.000450 YES RMS Force 0.000009 0.000300 YES Maximum Displacement 0.000023 0.001800 YES RMS Displacement 0.000017 0.001200 YES
The optimisation file is liked here
Frequency Analysis
Expected modes- (3X4)-6=6
Degenerate modes- none
Bending vibrations- modes 1 to 4
Bond stretch vibrations- modes 5 and 6
Symmetric- none
Number of bands expected in an experimental spectrum- 6 however some are very close and may merge. On the predicted spectrum from Gaussian 5 resolved peaks can be seen.
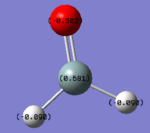
Charge Analysis
Charge on the O-atom- -0.502
Charge on the H-atom- -0.090
Charge on the Si-atom- +0.681