Rep:Mod:pz1122
Conformational analysis using Molecular Mechanics (Part 1)
The hydrogenation of cyclopentadiene dimer
Kinetic or Thermodynamic control?
Cyclopentadiene molecules readily dimerizes into the following two molecules 1 and 2, and hydrogenation of one of the dimer affords one of the dihydro derivatives 3 or 4 initially (Fig.1):
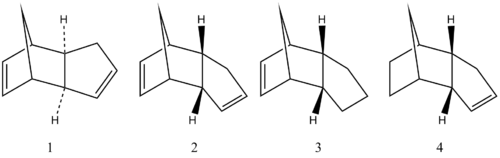
From basic theory of organic chemistry, one diene and a dienophile reacts readily under room temperature to give a dimer via a reversible Diels-Alder cycloaddition. The transition state of this reaction shows secondary orbital overlap between conjugated π orbitals of diene and π* orbitals of dienophile, only if the final dimer is endo (molecule 2 above). As predicted, this extra orbital overlap further stabilizes the transition state, hence lowers the activation energy, resulting in kinetic product 2. Experimental evidences have showed that endo product is especially preferred over another exo product 1. To explain this behaviour and to investigate whether the dimerization is under thermodynamic or kinetic control, calculations of molecular energies was performed using Avogadro with MMFF94s force field. Extra calculations was carried out using ChemBio3D to make a reasonable comparison. The results will be collected in a table and treated later.
The endo dimer can be hydrogenated resulting into two new molecules 3 and 4. They will be fully hydrogenated after a long time. It is our interest to discover which one of the hydrogenated molecule, 3 or 4, is more thermodynamically stable, and whether the reaction is under thermodynamic or kinetic control.
First, in the table below, information was collected for each molecule via Avogadro (MMFF94s force field):
| Property | Dimer 1 | Dimer 2 | Dimer 3 | Dimer 4 |
|---|---|---|---|---|
| Bond Stretching Energy | 3.54298 | 3.46742 | 3.30582 | 2.82315 |
| Angle Bending Energy | 30.77292 | 33.19132 | 30.87750 | 24.68533 |
| Stretching Bending Energy | -2.04139 | -2.08212 | -1.92614 | -1.65720 |
| Torsional Energy | -2.73101 | -2.94945 | 0.07177 | -0.37840 |
| Out-of-plane Bending Energy | 0.01486 | 0.02197 | 0.01434 | 0.00028 |
| van der Waals Energy | 12.80137 | 12.35726 | 13.25868 | 10.63731 |
| Electrostatic Energy | 13.01372 | 14.18430 | 5.12088 | 5.14702 |
| Total Energy | 55.37344 kcal/mol | 58.19070 kcal/mol | 50.72286 kcal/mol | 41.25749 kcal/mol |
Meanwhile, I also put the optimized geometry of each molecule:
| Dimer 1 | Dimer 2 | Dimer 3 | Dimer 4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Exo dimer 1
|
Endo dimer 2
|
Hydrogenated dimer 3
|
Hydrogenated dimer 4
|
Alternative calculations were performed using ChemBio3D with MM2 force fields:
| Property | Dimer 1 | Dimer 2 | Dimer 3 | Dimer 4 |
|---|---|---|---|---|
| Stretch | 1.2852 | 1.2516 | 1.2348 | 1.0962 |
| Bend | 20.5794 | 20.8470 | 18.9297 | 14.5267 |
| Stretch-Bend | -0.838 | -0.8361 | -0.7606 | -0.5491 |
| Torsion | 7.6558 | 9.5108 | 12.1326 | 12.4972 |
| 1,4 VDW | 4.2329 | 4.3188 | 5.7306 | 4.5127 |
| Dipole | 0.3775 | 0.4474 | 0.1631 | 0.1406 |
| Total Energy | 31.8765 kcal/mol | 33.9976 kcal/mol | 35.9266 kcal/mol | 31.1521 kcal/mol |
Analysis
First we look at the energy profiles obtained from Avogadro. The calculated total energies indicate that exo dimer is more thermodynamically stable than endo dimer. It is noticed that a slightly large difference in terms of angle bending energy between two molecules compared to other energy terms, and this energy difference largely contributes to the thermodynamic stability of exo dimer. According to the fact that it is the endo dimer, rather that the exo dimer will be our final product in dimerization, we can justify that the D.A. reaction is generally under kinetic control, rather than thermodynamic control. The endo reaction pathway has a lower kinetic barrier, i.e. activation energy, than the exo pathway. This is mainly because of secondary orbital overlap of the endo transition state, as it is mentioned above. While in exo transition state, there is no such extra stabilization.
Under MM2 force fields, we can still find that endo dimer is higher in energy. What looks different is the energy contributions. Torsional strain now plays a more significant contribution in the total energy difference. The close approach of the two rings in endo dimer may give rise to steric clashes.
Second, different regioselectivity of hydrogenation afford two dimers 3 and 4, and we found that dimer 4 is more T.D. stable than 3. And it can be recognized that bending energy and van der Waals energy of 3 are much higher than those of 4. This indicates the hydrogenated dimer 3 suffers from more ring strain in a thermodynamic sense. Assuming that the hydrogenation is under kinetic control (transition state stability), product 3 should come out initially. Only after a longer time will product 4 come out. Thus, we conclude that hydrogenation of 2 to 3 is much easier than 2 to 4, despite that 4 is much lower in energy than 3.
In the total synthesis of Taxol, two key intermediates reported by Paquette [1], 9 and 10, was originally synthesised with the carbonyl group pointing either up or down, as shown in Fig.2. One of the isomer will automatically transform into another isomer with different carbonyl positions. It was also noted that certain functionalisation of the alkene was quite slow.
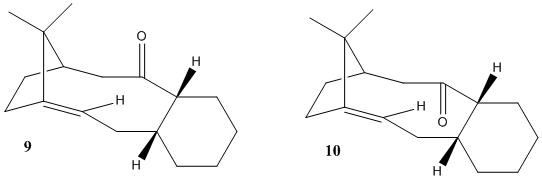
Here we want to explore their stability as well as difference in reactivity by carrying out calculations using Avogadro and MMFF94s force-field. Results are shown below.
Optimized molecules
| Intermediate 9 | Intermediate 10 | ||||||
|---|---|---|---|---|---|---|---|
|
|
Relative Energies
| Property | Intermediate 9 | Intermediate 10 |
|---|---|---|
| Bond Stretching Energy | 7.65321 | 7.70163 |
| Angle Bending Energy | 28.26801 | 16.86358 |
| Stretching Bending Energy | -0.082 | -0.22900 |
| Torsional Energy | 0.31236 | 2.07824 |
| Out-of-plane Bending Energy | 0.97223 | 0.83245 |
| van der Waals Energy | 33.11723 | 34.01453 |
| Electrostatic Energy | 0.30508 | -0.06754 |
| Total Energy | 70.54611 kcal/mol | 61.19390 kcal/mol |
Results analysis
I find that intermediate 10 is more stable than 9, and the huge difference in bending energies between two molecules contribute to the total energy differences. I have managed to measure the O-C-C bond angles, and noticed that the angles in molecule 10 is slightly larger than that in molecule 9. Thus, I interpret this as the C=O group in 10 will have less steric clash with the ring system, resulting in lower bending energy. Interestingly, I also found a more stable conformation of 9, which has a total energy of 60.682 kcal/mol, but I cannot justify whether this conformer is correct.
| Intermediate 9 | |||
|---|---|---|---|
|
Obviously, this conformer is only slightly more stable than molecule 10. And under MMFF94s force field, this conformer and molecule 10 do not show much difference in erergy. A lack of supporting literature references makes the comparisons unreliable.
Considering the reactivity of alkene functional group, the extra stability is possibly due to the alkene 'bridgehead' two large rings, which is called 'hyperstability'[2]. Hyperstability refers to the less in torsional strain than the parent hydrocarbon, and there is a term especially associated with it,called Olefinic Strain. It is calculated by taking the difference between the total strain energy of the most stable parent hydrocarbon and the total strain energy of the olefin, also in its most stable conformation. And it was also reported that if the calculated olefinic strain is smaller 17 kcal/mol, double bond will be kinetically stable at room temperature. If its value is larger than 17 kacl/mol, the olefin will not be isolable, and only certain type of spectroscopy techniques can allow us to trap those intermediates.
In all the cases above, the cyclohexane rings all adopts the most stable conformations--chair conformation. Thus I suggested that by twisting the ring into different shapes, it is possible to get conformations of higher energy, which we should be aware of.
Spectroscopic Simulation using Quantum Mechanics
Here we want to investigate how our predicted 1H and 13C NMR spectra of one of the two molecules 17 and 18 (Fig.3) agree with the literature reported values. The computer modelling was performed by Gaussian and geometry optimization was necessary.
Two molecules
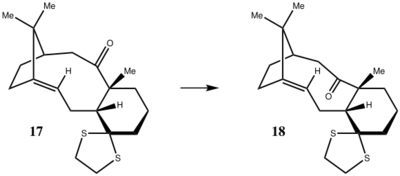
And we have selected molecule 17 to investigate: (optimized under MMFF94s force fields)
Taxol Intermediate 9 |
Results
The predicted 1H spectrum(Fig.4) using TMS B3LYP/6-31G(d,p)as a reference and chloroform as a solvent, was posted below, and a table comparing the chemical shift with the literature was also presented:
| Literature (ppm) | Molecule 17 (ppm) |
|---|---|
| 4.84 (dd,1H) | 5.10 (1H) |
| 3.40-3.10 (m, 4H) | 3.29 (1H) |
| 2.99 (dd, 1H) | 3.15 (4H) |
| 2.80-1.35 (series of m, 14H) | 2.94 (1H) |
| 1.38 (s, 3H) | 2.68 (2H) |
| 1.25 (s, 3H) | 2.60 (1H) |
| 1.10 (s, 3H) | 2.41 (3H) |
| 1.00-0.80 (m, 1H) | 2.27 (2H) |
| 2.11 (2H) | |
| 2.02 (1H) | |
| 1.95 (1H) | |
| 1.73 (1H) | |
| 1.67 (1H) | |
| 1.58 (2H) | |
| 1.44 (1H) | |
| 1.03 (2H) | |
| 0.89 (4H) | |
| 0.58 (1H) |
From the computed NMR specturm, we can notice a significant deviation from experimentally measured chemical shift values. For example, the last chemical shift of a proton has shifted to high field about 0.2 ppm. Considering that the NMR prediction mehtod is quite sensitive to different conformations, we can say that our prediction is acceptable. The difference may due to the conformation we are using here is not exactly the same as the one in literature. Unfortunately, the prediction method we use here do not include any information regarding the multiplicity and coupling constants. But we are still be able to calculating the coupling constants, and it is a very time-consuming process. In general, our prediction showed a satisfactory agreement with the literature reported values.
Detailed assignments of the predicted spectrum is given below with Fig.5 to help:
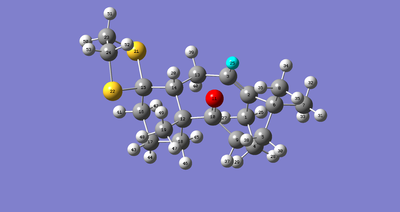
| chemical shift/ppm | atom |
|---|---|
| 5.10 | 25 |
| 3.29 | 52 |
| 3.15 | 53,50,51,39 |
| 2.94 | 20 |
| 2.68 | 27,36 |
| 2.60 | 42 |
| 2.41 | 38,37,40 |
| 2.27 | 28,26 |
| 2.11 | 45,30 |
| 2.02 | 41 |
| 1.95 | 48 |
| 1.73 | 43 |
| 1.67 | 44 |
| 1.58 | 29,33 |
| 1.44 | 46 |
| 1.03 | 49 |
| 0.89 | 34,32,31,35 |
| 0.58 | 47 |
Next, the 13C NMR(Fig.6) was compared with literature and assigned(Fig. 7).
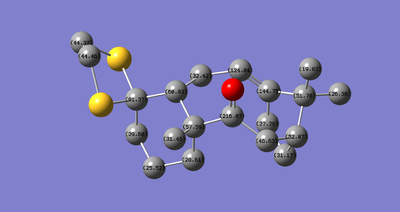
| Literature (ppm) | Molecule 17 (ppm) |
|---|---|
| 218.79 | 216.07 |
| 144.63 | 144.75 |
| 125.33 | 124.84 |
| 72.88 | 91.37 |
| 56.19 | 60.83 |
| 52.52 | 57.59 |
| 48.50 | 52.87 |
| 46.80 | 51.70 |
| 45.76 | 46.63 |
| 39.80 | 44.46 |
| 38.81 | 44.32 |
| 35.85 | 39.60 |
| 32.66 | 32.42 |
| 28.79 | 31.46 |
| 28.29 | 31.17 |
| 26.88 | 28.61 |
| 25.66 | 27.26 |
| 23.86 | 26.30 |
| 20.96 | 25.52 |
| 18.71 | 19.82 |
When checking the predicted chemical shift, spin-orbit coupling errors of the carbons connecting to heavy atoms(sulphur in this molecule) must be taken into account. Typically, C-Cl bonds need to be corrected by -3 ppm[4]. Since sulphur has nearly the same molecular weight as chloride, I assume that C-S bonds also need to be corrected by -3 ppm. After such corrections, except the carbon with 91 ppm, all the remaining carbons show good agreement with the experimental values. Thus, we can conclude that, from calculating the 13C NMR spectrum, the experimental spectrum was correctly assigned.
References for this part
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- ↑ W. F. Maier, P. Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103, 1891. DOI:10.1021/ja00398a003
- ↑ 3.0 3.1 Spectroscopic data: L. Paquette, N. A. Pegg, D. Toops, G. D. Maynard, R. D. Rogers, J. Am. Chem. Soc.,, 1990, 112, 277-283. DOI:10.1021/ja00157a043
- ↑ C. Braddock and H. S. Rzepa, J. Nat. Prod., 2008, 71, 728-730. DOI:10.1021/np0705918
Analysis of the properties of the synthesised alkene epoxides (Part 2)
Two catalytic systems
Here we want to investigate the structures of two chiral catalysts we used for asymmetric epoxidation of olefins. The Conquest program was used to search for the structure in Cambridge Crystal Database (CCDC), and the crystal structure was analyzed using Mercury program. The epoxidation process with Shi catalyst is shown below:
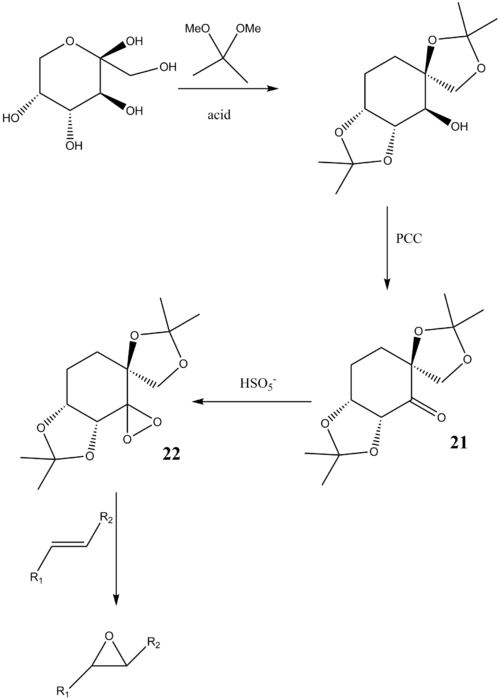
And the epoxidation of olefins using Jacobsen catalyst is shown below:
Both processes have been reported by Shi et al[1] and Jacobsen et al[2],[3] respectively.
Crystal structure of Shi and Jacobsen Catalyst
Following crystal structure of Shi and Jacobsen catalyst was found via CCDC, and we especially want to look at the difference between two anomeric centers:
| Shi catalyst | Jacobsen catalyst | ||||||
|---|---|---|---|---|---|---|---|
|
|
When analyzing the structure of Shi catalyst, we have to pay special attention to the anomeric centers (i.e. those with O-C-O structures). The average C-O bond length near the anomeric center is about 0.141 nm, as we measured from the crystal structures. Compared to the normal C-O bond length which is around 143 pm, it is slightly shorter. This is mainly due to the anomeric effect. When the central carbon and two adjacent oxygen atoms are orthogonal to each other, the lone pair on one of the oxygen is able to donate into the empty C-O σ* anti-bonding orbital and provide extra stability of the C-O bond. Therefore, one of the C-O bond is strengthened, another is weakened by additional electron density on anti-bonding orbital. In average, we should observe a slight decrease in bond length.
In terms of Jacobsen catalyst, an important information that worth noticing is the close approach of two tertiary butyl groups, as indicated above. The distance between two approaching hydrogens is measured, which are 0.242 nm, 0.264 nm, 0.282 nm and 0.275 nm. These values are roughly equal to the van der Waals distance for two hydrogen, which is about 0.240 nm[4] and we can soon interpret this as an weak attractive interactions between two approaching hydrogen atoms. As a result, this weak interactions stop the alkene from approaching the metal center from that bulky face, giving rise to a stereospecific epoxide.
NMR properties of epoxide product
NMR spectra of two epoxides, styrene oxide and trans-stilbene oxide was caluclated. The geometry of the two alkene epoxides was optimized using Avogadro under MMFF94s force fields.


| styrene oxide | trans-stilbene oxide | ||||||
|---|---|---|---|---|---|---|---|
|
|
The chemical shift of each hydrogen is shown on the atoms in the graph on the right, and 13C NMR spectra was assigned as well.
We notice that NMR is unable to give an assignment of the stereochemistry of our epoxide product.
Assigning the absolute configuration of the product
For the two epoxides above, the stereochemistry can be determined exactly by calculating the chiroptical properties for a specified enantiomer. Here we use the Cambridge variation on the B3LYP density functional method to calculate the optical rotation at a specified wavelength of light. Also, we compare the calculated value with literature reported optical rotations to see whether there is a match. The results is summarized below:
| Properties | (R)-styrene oxide | (R,R)-trans-stilbene oxide |
|---|---|---|
| optical rotation at 589 nm(Calculated) | -30.31 deg. | 298.22 deg. |
| optical rotation at 589 nm(literature reported) | -33.3 deg.[5] | 250.8 deg.[6] |
We can notice that calculated optical rotation values are close to the literature reported values, which means that we can assign each of our enantiomer with a specific stereochemistry.
Alternatively, I also performed a vibrational circular dichroism calculation on (R)-styrene oxide and (R,R)-trans-stilbene oxide, and the following spectra are obtained:
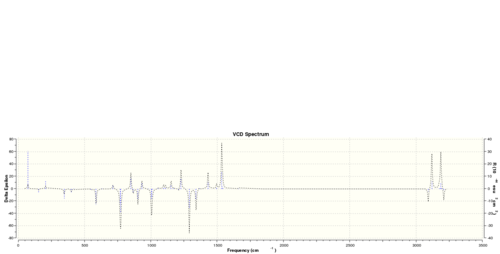
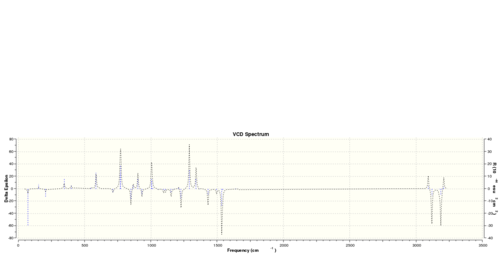
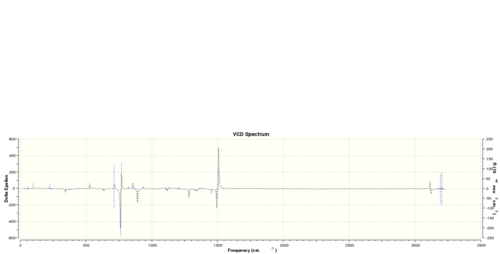
Obviously, the R and S enantiomer have completely symmetrical VCD spectrum.
Using the (calculated) properties of transition state for the reaction (β-methyl styrene only)
The selectivity of the catalyst on different substrates β-methyl styrene can be calculated via transition states. This was achieved involving the total energy for each system as corrected for zero-point energy and entropy. We need to find the transition state with the lowest energy, and calculate the difference between the free energy of each enantiomer. Then, using this fomula,
we can know the value of K which corresponds to the ratio of two enantiomers.
Results for Jacobsen epoxidation of β-methyl styrene is given below:
| ΔG(trans-β-methyl styrene epoxidation) (kJ/mol) | K(trans-β-methyl styrene) | ΔG(cis-β-methyl styrene epoxidation) (kJ/mol) | K(cis-β-methyl styrene) |
|---|---|---|---|
| -21.36369041 | 5533.053747 | -22.31412127 | 8118.624166 |
Clearly, we can justify that Jacobsen catalyst is about 1.5 fold more selective with cis alkene than with trans alkene, because the value of K for cis alkene is significantly higher. In other words, the epoxidation using Jacobsen ligand with cis-olefin has a ee value about 99%, and the reaction is predicted to give exclusively (S,R)-cis-beta-methyl styrene oxide. This is a rather high enantioselective reaction! If we check the literature value, Jacobsen et al reported the selectivity to be about 92%[7]. I think that different solvent systems used may change the ee value. In our calculated case, water is used as a solvent, while in the literature was not.
I also calculated the ΔG of Shi-catalysed trans-β-methyl styrene epoxidation to be -20.21897257 kJ/mol, and the corresponding K value is 3486.64589. This means that the epoxidation reaction will exclusively give (R,R)-trans-beta-methyl styrene oxide, with ee value close to 99%. Due to a lack of information, I cannot compare the selectivity between cis olefin and trans olefin using Shi as a epoxidation catalyst.
Investigating the non-covalent interactions in the active-site of the reaction transition state
Non-covalent interactions (NCI) include hydrogen bonds, electrostatic attractions and dispersion-like close approaches of pairs of atoms. It allows us to find the electron density and its curvatures. I have used the transition state for the Shi epoxidation of (R)-trans-β-methyl styrene for electron density computation and NCI analysis. The results are shown below:
NCI |
The colour indicates whether the interaction is attractive or repulsive. Blue means very attractive, green is mildly attractive, yellow is mildly repulsive and red is strongly repulsive. Normally, we need to ignore the bond formed in a transition state, which is a red circle. And we can find there is strong attractive interactions between the catalyst and the branching alkyl group on the substrate. We may also notice the red region between two acetal oxygens, which indicates a strong repulsive interaction, possibly due to repulsions between lone pairs on oxygens as they are so close together. Overall, this transition state is rather stable due to large favourable attractive interactions that lowers the overall energy.
Investigating the Electronic topology (QTAIM) in the active-site of the reaction transition state
This part is complementary to the NCI analysis, as it focuses more on the electron density in the covalent regions of the molecule. Here I chose the same transition state as the NCI analysis, and performed a QTAIM analysis.
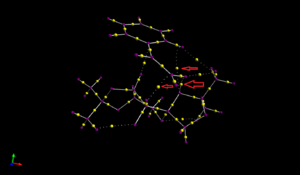
All the yellow points can be classified as a BCP (bond critical point). There are two types of BCP, one is covalent and another is non-covalent. The red arrows on the graph shows three interesting weak non-covalent BCP, where oxygen interacts with ring hydrogen. They are not associated with covalent bonds, but with strong attractive interactions between oxygen and the ring. This shows that in the transition state, covalent interactions start to form between oxygen and hydrogen.
Suggesting new candidates for investigations
I have found the following molecule,cis R-(+)-pulegone oxide, with optical rotation 853.9 deg[8] in ethanol at 324 nm, can be our new candidate. This molecule contains two oxygen and one tri-substituted cyclohexanone, which are both rather simple structure. I believe it is commercially available, and it will be interesting to investigate into its optical rotation and different conformations, especially the cyclohexane ring.
new candidate |
References for this part
- ↑ O. A. Wong , B. Wang , M-X Zhao and Y. Shi J. Org. Chem., 2009, 74, 335–6338; DOI:10.1021/jo900739q
- ↑ E. N. Jacobsen , W. Zhang , A. R. Muci ,J. R. Ecker , L. Deng J. Am. Chem. Soc., 1991, 113, 7063–7064; DOI:10.1021/ja00018a068
- ↑ M. Palucki , N. S. Finney , P. J. Pospisil , M. L. Güler , T. Ishida , and E. N. Jacobsen, J. Am. Chem. Soc., 1998, 120, 948–954; DOI:10.1021/ja973468j
- ↑ DOI:10.1021/jp8111556
- ↑ DOI:10.1021/ja00853a029
- ↑ DOI:10.1039/B606881B
- ↑ E. Jacobsen, W. Zhang, A. Muci, J. Ecker and L. Deng,"Highly enantioselective epoxidation catalysts derived from 1,2-diaminocyclohexane", J. Chem. Educ., 1991, 113(18), 7063-7064; DOI:10.1021/ja00018a068
- ↑ DOI:10.1021/jo01045a016
