Rep:Mod:psl1618
EX3 section
BH3
B3LYP/6-31G level
- Energy = -26.61532 a.u. (5 d.p) In comparison, total energy using 3-21G basis set was -26.46226 a.u. (5 d.p)
Item Value Threshold Converged? Maximum Force 0.000014 0.000450 YES RMS Force 0.000009 0.000300 YES Maximum Displacement 0.000057 0.001800 YES RMS Displacement 0.000037 0.001200 YES
Link to frequency log file: File:PSL BH3 FREQ.LOG
Low frequencies --- -10.0419 -2.9960 -0.0055 0.4925 2.1764 3.7030 Low frequencies --- 1162.9539 1213.1540 1213.1567
Optimized BH3 structure |
Vibrational spectrum for BH3
| wavenumber (cm-1) | Intensity (arbitrary units) | Symmetry | IR active? | Type |
| 1162.95 | 92.5526 | A1 | Yes | Out-of-plane Bend |
| 1213.15 | 14.0531 | E | Yes (slightly) | Bend |
| 1213.16 | 14.0568 | E | Yes (slightly) | Bend |
| 2582.42 | 0 | A1 | No | Symmetric Stretch |
| 2715.61 | 126.3250 | E | Yes | Asymmetric Stretch |
| 2715.61 | 126.3155 | E | Yes | Asymmetric Stretch |
IR Spectrum for BH3
Although there are 6 vibrations, there are only 3 peaks in the IR spectrum. This is because the asymmetric stretches and bends at 1162 and 2715 cm-1 respectively are both degenerate, so only 2 peaks are observed at those frequencies. The third peak is that of the out-of-plane bend. The symmetric stretch is not IR active since there is no change in dipole moment, so no peak is observed.
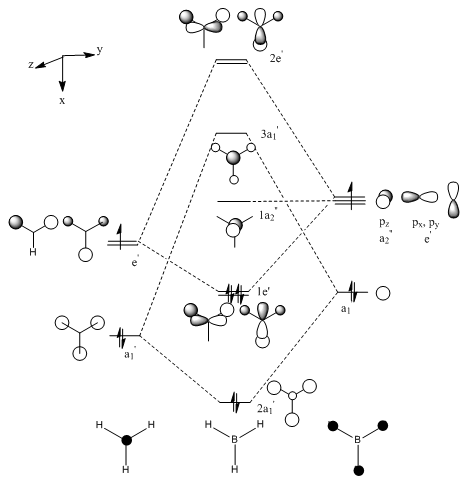
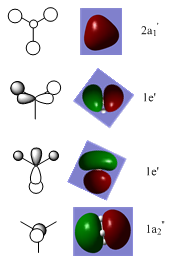
Molecular Orbitals
The molecular orbital diagram for BH3 is as follows:
The key occupied orbitals, the HOMO and the LUMO predicted using Gaussview are compared to those predicted using LCAO theory:
The overall shape, size and phases of the calculated MOs correspond well with those predicted in the MO diagram, showing that for BH3, LCAO theory gives a very good approximation of the molecular orbitals. However, this is probably because B and H are light atoms and the molecule only has 8 electrons, meaning that there aren't significant relativistic effects.
Ammonia-Borane Association Energy
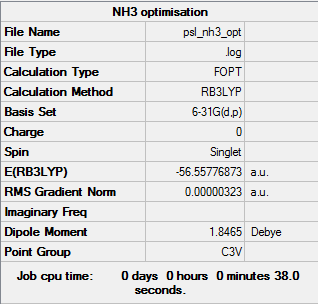
Key data for Ammonia
B3LYP/6-31G level
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES
Link to frequency log file: File:PSL NH3 FREQ.LOG
Low frequencies --- -8.5646 -8.5588 -0.0044 0.0454 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
Optimised structure for NH3 |
Key data for Ammonia-Borane
B3LYP/6-31G level
Item Value Threshold Converged? Maximum Force 0.000122 0.000450 YES RMS Force 0.000058 0.000300 YES Maximum Displacement 0.000513 0.001800 YES RMS Displacement 0.000296 0.001200 YES
Link to frequency log file: File:PSL BH3NH3 FREQ 1.LOG
Low frequencies --- -0.0010 -0.0006 0.0007 17.5817 27.9112 40.2360 Low frequencies --- 266.5197 632.3594 639.4724
It is noted that the energy given in the summary table after the frequency calculation is -83.22469057 au, which differs from that of the optimisation calculation by the last 4 decimal places. However, the 'Item table' of the frequency calculation shows that the structure is optimised:
Item Value Threshold Converged? Maximum Force 0.000113 0.000450 YES RMS Force 0.000061 0.000300 YES Maximum Displacement 0.000660 0.001800 YES RMS Displacement 0.000442 0.001200 YES
Optimised structure for NH3BH3 |
Calculation of the association energy of the B-N Bond:
- E(NH3): -56.55777 au
- E(BH3): -26.61532 au
- E(NH3BH3): -83.22469 au
- ΔE = E(NH3BH3)-[E(NH3)+E(BH3)] = -83.22469 - (-56.55777 - 26.61532) = -0.05160 au (5 d.p) = -135 kJ/mol
Smf115 (talk) 08:33, 17 May 2018 (BST)Correct calculation but a comment on the result with comparison to other bond dissociation energies was required.
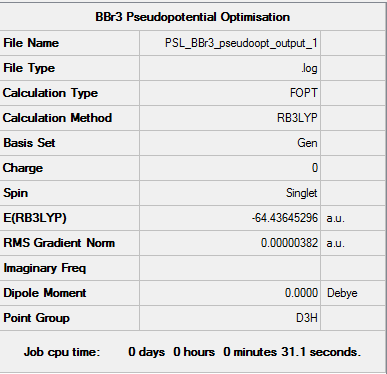
BBr3 pseudopotential optimisation
To carry out the optimisation, the GEN basis set was used - B3LYP/6-31G level was used for B and pseudo-potential LanL2DZ for Br
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000023 0.001200 YES
Link to frequency optimisation file: DOI:10042/202320
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
Optimized BBr3 structure |
Project section
For all the following optimisations, a mixed between pseudo-potentials and basis sets were used via the GEN function. Al and Cl atoms were optimised using B3LYP/6-31G(d,p) and Br atoms were optimised using LanL2DZ pseudo-potentials.
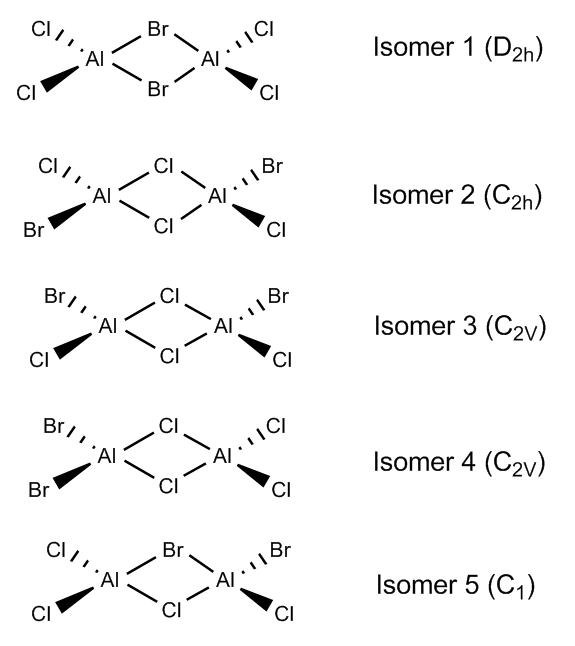
Possible Isomers of AlBr2Cl4 and their symmetries
Isomer 1: Bridging Bromides
Energy of isomer = -2352.40631 a.u. (5 d.p) = -6175067 kJ/mol (nearest integer)
Item Value Threshold Converged? Maximum Force 0.000003 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000040 0.001800 YES RMS Displacement 0.000015 0.001200 YES
Link to frequency file:File:Psl brbridge freq output.log
Low frequencies --- -5.1748 -5.0356 -3.1468 0.0027 0.0031 0.0034 Low frequencies --- 14.8260 63.2702 86.0770
Optimized Isomer 1 structure |
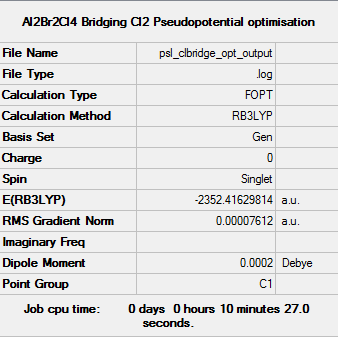
Isomer 2: Bridging Chlorides
Energy of isomer = -2352.41630 a.u. (5 d.p) = -6175093 kJ/mol (to nearest integer). This shows that the isomer with the bridging Cl ions is the more stable conformer. This is as expected since Al and Cl are both in the third row, so their orbitals are of more similar size and energy compared to those of Br, leading to more overlap. This means that the Cl lone pairs are more able to relieve Al's electron deficiency, leading to a structure that is overall more stable.
Item Value Threshold Converged? Maximum Force 0.000171 0.000450 YES RMS Force 0.000053 0.000300 YES Maximum Displacement 0.001613 0.001800 YES RMS Displacement 0.000674 0.001200 YES
Link to frequency file:File:Psl clbridge freq output.log
Low frequencies --- -6.1262 -2.0171 -0.0014 0.0026 0.0032 1.3830 Low frequencies --- 17.8118 49.0588 72.9995
Optimized Isomer 2 structure |
AlCl2Br Monomer
Energy of monomer = -1176.19014 a.u. (5 d.p) = -3087499 kJ/mol (to nearest integer)
Item Value Threshold Converged? Maximum Force 0.000136 0.000450 YES RMS Force 0.000073 0.000300 YES Maximum Displacement 0.000681 0.001800 YES RMS Displacement 0.000497 0.001200 YES
Link to frequency file:File:Psl monomer freq output.log
Low frequencies --- 0.0039 0.0043 0.0050 1.3569 3.6367 4.2604 Low frequencies --- 120.5042 133.9178 185.8950
Optimized AlCl2Br structure |
Calculation of the dissociation energy of Isomer 2
- E(Isomer) = -6175093 kJ/mol
- E(Monomer) = -3087499 kJ/mol
- Dissociation energy = [2 x E(Monomer)] - E(Isomer) = 95 kJ/mol
- Since the value is positive, the dimer is more stabilised than the isolated isomers, which is as expected since the dimerisation relieves the electron deficiency of the Al atoms.
Smf115 (talk) 08:39, 17 May 2018 (BST)Correct calculation and consideration given to the accuracy when reporting the result.
Molecular orbitals for Isomer 2
MO 38 (Symmetry: Ag)
MO 43 (Symmetry: Bu)
MO 54 (HOMO, symmetry: Bu)
Smf115 (talk) 08:37, 17 May 2018 (BST)Good MO analysis and nice inclusion of the FO's too. The LCAOs have clear annotations and the nodal planes, strength and character of the interactions are identified and explained. To improve subtler points could have been made, such as the size difference in contributions from Br and Cl in MO 54.
Smf115 (talk) 08:37, 17 May 2018 (BST)Overall a clear report with a very good project section but some missing information and errors throughout the first section.