Rep:Mod:pretty shitty city
Molecular Mechanics Modelling
Molecular Mechanics is a method of 3D molecular modelling which is used to optimise the molecular geometry of a configuration by minimising the calculated total energy of the molecule. The total energy of a configuration consists of the following:
1. The sum of all diatomic bond stretches (each expressed as a simple Hooke's law potential). 2. The sum of all triatomic bond angle deformations (also a simple Hooke's law potential) 3. The sum of all tetra-atomic bond torsions (a cosine dependance) 4. The sum of all non-bonded Van der Waals repulsions (using a simple 6/12 potential). 5. The sum of all electrostatic attractions of individual bond dipoles.
In this exercise, the Allinger MM2 and MMFF94 molecular mechanics models will be implemented using ChemBio3D Ultra to determine the optimum geometry and energy minima for particular configurations, and hence to explain reaction mechanisms and issues of selectivity.
The Hydrogenation of Cyclopentadiene Dimer
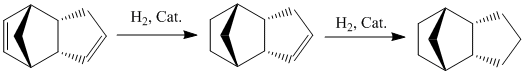
Dimerisation of cyclopentadiene
Cyclopentadiene dimerises readily and rapidly at room temperature via a Diels-Alder mechanism. It was shown by Wilson et al in 1944 that the reaction specifically produced the endo dimer 2, with no formation of the exo dimer 1.
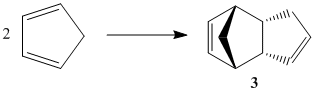
The relative energies of the dicyclopentadiene dimers were calculated using the MM2 force field. These were compared in the table below, determining their relative stabilities.
| Dimer | Energy/ kJ mol-1 | ||
|---|---|---|---|
| exo-dicyclopentadiene 1
|
133.4 | ||
| endo-dicyclopentadiene 2
|
142.4 |
As shown in the above table, the exo dimer is thermodynamically more stable than the endo dimer, making the exo dimer the favoured product under thermodynamic control. Herndon et al experimentally observed the exo dimer to be the lower energy species in agreement with the molecular mechanics model. The Diels-Alder reaction is irreversible, hence the endo dimer is produced via kinetic control. Caramella et al reported the reaction resulting in the formation of the endo dimer proceeds via a lower energy bispericyclic transition structure. This relatively low activation energy reaction can therefore proceed rapidly, and under kinetic control, the endo product predominates.
The relative stability of the endo transition structure can be explained by Alder's endo addition rule, where there is maximum accumulation of unsaturated centres.
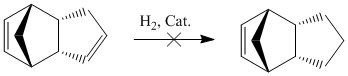
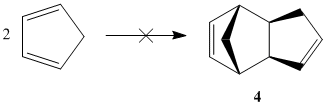
Hydrogenation of cyclopentadiene dimer
Hydrogenation of endo-dicyclopentadiene (see above) initially results in the formation of the more stable dihydro derivative 4. After prolonged reaction, the respective tetrahydro derivative forms.
The relative energies of the dihydro derivatives were calculated using the MM2 force field. These were compared in the table below, determining their relative stabilities.
| Hydrogenation Product | Stretching/ kJmol-1 | Bending/ kJmol-1 | Torsion/ kJmol-1 | van der Waals/ kJmol-1 | Hydrogen Bonding/ kJmol-1 | Total Energy/ kJmol-1 | ||
|---|---|---|---|---|---|---|---|---|
| 3
|
5.3 | 82.9 | 45.5 | 23.6 | -0.7 | 149.4 | ||
| 4
|
4.5 | 60.8 | 52.3 | 18.9 | -0.6 | 130.5 |
The total energy of 3 (149.4 kJmol-1) is significantly higher than that of 4 (130.5 kJmol-1). The most significant contribution to this higher energy originates from the higher Bending energy of 3 (82.9 c.f. 60.8 kJmol-1).
The bending energy is the energy associated with deforming bond angles from their preferred values. The location of the double bond in this C10H14 species clearly has a large impact on its thermodynamic stability. In product 3, the two carbon atoms attached directly to the C=C double bond are bridged by a methylene carbon. This bridging effect brings the bridgehead carbons closer together, thereby forcing the C=C-C angle to reduce from the preferred 120o for sp2-hybridised systems to a calculated 107.8o. In product 4 however, the C=C double bond is part of a five-membered ring with no adjacent bridges. The bridging effect from above is not a factor in 4 therefore, and two C=C-C bond angles are calculated to be 112.4o and 113.1o. Hence, 4 has a smaller Bending Energy and therefore a smaller Total Energy. This conclusion is backed up by Zou et al with DFT calculations. In summary, 4 is the thermodynamic product, and hence, this reaction is under thermodynamic control.
Stereochemistry of Nucleophilic Additions to a Pyridinium Ring (NAD+ analogue)
The stereochemistry of nucleophilic additions to a pyridinium ring (NAD+ analogue) will be investigated using ChemBio3D Ultra.
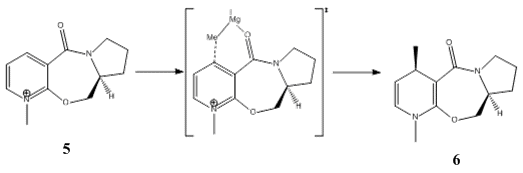
Reaction of Pyridinium ring in N-methyl pyridoxazepinone with Grignard reagent
Nucleophilic addition of Grignard reagents to N-methyl salts of pyridoxazepinone are highly regioselective and stereoselective Methyl magnesium iodide reacts with N-methyl pyridoxazepinone according to the following reaction scheme.
Shultz et al postulated that the electropositive Mg atom of MeMgI coordinates to the electronegative oxygen atom of the amide functional group, thereby stabilising the transition state and directing the attack of the Grignard reagent. This probably takes place via the formation of a six-membered ring as shown in the diagram. The methyl group is directed to the face of the aromatic ring which comes out of the plane of the screen, allowing conjugate nucleophilic addition and hence dictating the stereochemistry and regiochemistry of the resulting addition product. The conformational stability of N-methyl pyridoxazepinone depends on the dihedral angle described by the C=O bond and the aromatic plane, and also on the local conformations of the 5- and 7-membered rings. The geometry was optimized using the MMFF94 force field and by tweaking the local conformations of the 5- and 7-membered rings. When an energy minimum was located, the total energy of various conformations (concentrating on the geometry of the C=O bond due to its influence on the stereochemical outcome of the reaction) were calculated using the MMFF94 force field and then compared.
The plot shows that there is an optimum dihedral angle of 9.4o (240.3 kJmol-1)where the C=O bond points slightly out of the plane of the screen in the above reaction scheme.
Coordination of the Mg atom to the carbonyl orientates and directs the Me group to attack at the C-4 position above the ring. This explains the regio- and stereoselectivity of the nucleophilic addition of methyl magnesium iodide to N-methyl pyridoxazepinone.
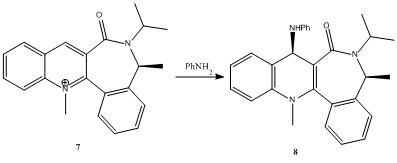
Reaction of Pyridinium ring in N-methyl quinolinium salt with aniline
The nucleophilic addition of aniline to N-methyl quinolinium salt is highly regio- and stereoselective.
Leleu et al observed that aniline, along with other bulky amines such as piperidine react on the opposite face of the amide "plane" with respect to the C=O bond. They concluded that the diastereofacial selectivity was a result of the steric control exerted by the C=O group i.e. the electrostatic repulsion between the nitrogen lone pairs and the carbonyl lone pairs.
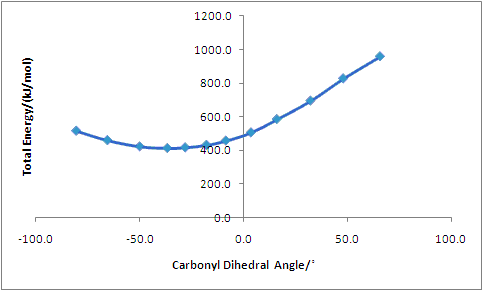
The plot shows that there is an optimum dihedral angle (lowest Total Energy value) of -36.7o (411.4 kJmol-1). This means that the carbonyl is pointing into the plane of the screen in the above reaction scheme. Attack by aniline, using the lone pair on the nitrogen atom, on the same face of the carbonyl would result in a very high energy transition state due to steric hindrance, and so the reaction does not proceed in this way. Instead, aniline attacks at the less hindered opposite side of the plane at the C-4 position. This explains the regio- and stereoselectivity of the addition of aniline to the N-methyl quinolinium salt.
Possible Improvements
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Paclitaxel(TAXOL) is a mitotic inhibitor used in cancer chemotherapy in the treatments of patients with lung, ovarian, breast cancer, head and neck cancer, and advanced forms of Kaposi's Sarcoma.
Atropisomerism
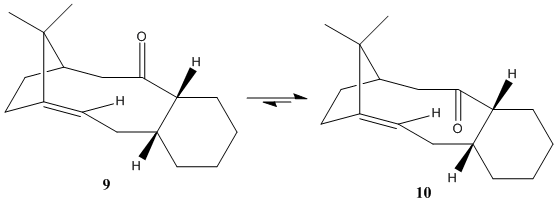
Atropisomers arise when an energy barrier exists between two different conformers due to sterically restricted rotation about a single bond. Paquette et al showed that an intermediate 9 involved in the synthesis of Taxol isomerises to intermediate 10
Using the MM2 force field, the energies of the atropisomers were calculated, and are compared in the following table.
| Intermediate | Stretching/ kJmol-1 | Bending/ kJmol-1 | Torsion/ kJmol-1 | van der Waals/ kJmol-1 | Hydrogen Bonding/ kJmol-1 | Total Energy/ kJmol-1 | ||
|---|---|---|---|---|---|---|---|---|
| 9
|
11.4 | 64.9 | 75.3 | 53.0 | -2.3 | 204.4 | ||
| 10
|
10.6 | 44.7 | 82.2 | 52.3 | -5.3 | 185.4 |
The atropisomer 9 (204.4 kJmol-1) is significantly less thermodynamically stable than 10 (185.4 kJmol-1). Again, this is mainly due to the Bending Energies of the respective species.
Looking at the C=C double bond, the two cis-related carbon atoms describe two separate C-C=C bond angles. Ideally (in an sp2 system) these angles would be 120o. In 9, these angles are 132.0o and 130.5o, whereas in 10 these angles are 127.1o and 124.1o. This means that there is a larger deviation from the optimum bond angle in 9 than there is in 10 and hence the Bending Energy of 9 is higher, and hence 9 has a higher Total Energy. A hyperstable alkene is one that is stable relative to its respective hydrogenation product. They were defined by Maier and Schleyer as alkenes which are less strained than their parent saturated hydrocarbons. Hence, the reaction which results in functionalisation of these C=C double bonds is slow. Using the MM2 force field, the energies of the atropisomers were calculated, and are compared in the following table.
| Hydrogenation Product | Stretching/ kJmol-1 | Bending/ kJmol-1 | Torsion/ kJmol-1 | van der Waals/ kJmol-1 | Hydrogen Bonding/ kJmol-1 | Total Energy/ kJmol-1 | ||
|---|---|---|---|---|---|---|---|---|
| 9H
|
12.8 | 75.0 | 91.2 | 71.3 | -3.0 | 250.7 | ||
| 10H
|
12.0 | 59.5 | 107.1 | 71.6 | -7.9 | 245.2 |
The hydrogenation products 9H and 10H are clearly less stable than their respective unsaturated species 9 and 10. It is said that the hyperstable alkenes have negative olefinic strain (OS) energies, which is defined as the extra strain associated with the double bond compared to the parent saturated species.
Semi-empirical Molecular Orbital theory modelling
Regioselective Addition of Dichlorocarbene
Orbital Control of Reactivity
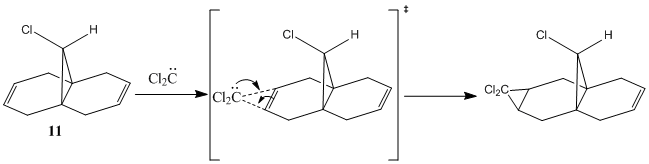
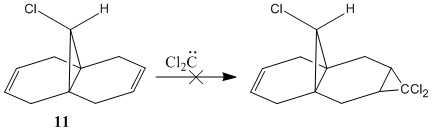
The geometry of compound 12 was first cleaned up with the MM2 force field. The "cleaned up" geometry of 12 was then treated by the MOPAC/PM6 MO method to give an approximate representation of the valence-electron molecular wavefunction. Representations of the LUMO+2, LUMO+1, LUMO, HOMO, and HOMO-1 are shown below.
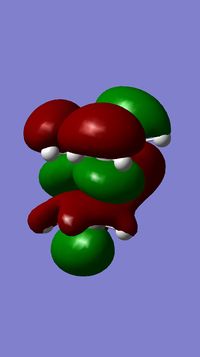
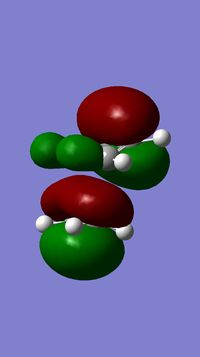
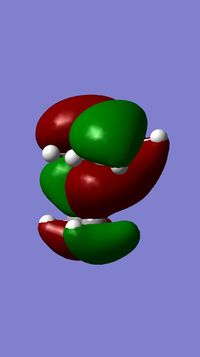
In the HOMO there is a higher electron density (greater orbital contribution) on the syn-alkene double bond than on the anti-alkene double bond. This suggests that the syn-alkene is more nucleophilic i.e. more susceptible to electrophilic addition. Hence, an electrophile, e.g. dichlorocarbene, would prefer to attack the electron-rich syn-alkene as opposed to the electron-deficient anti-alkene.
Rzepa et al attributed the extra stability of the anti-alkene to a favourable anti-periplanar overlap between the σ*C-Cl orbital (LUMO+1) and the anti-alkene πC=C orbital.
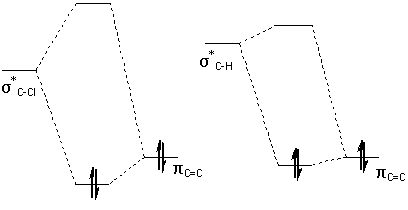
Vibrational Frequencies
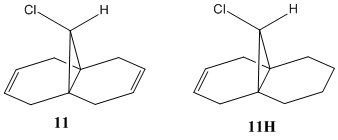
| System | syn C=C stretch/ cm-1 | anti C=C stretch/ cm-1 | C-Cl stretch/ cm-1 |
|---|---|---|---|
| Diene 11 | 1757.44 | 1737.03 | 770.84 |
| Alkene 11H | 1758.05 | - | 774.97 |
As shown in the above table, the calculated stretching frequency of the C-Cl bond in both 11 and 11H are in agreement with the literature value of 780 cm-1. The C-Cl bond clearly strengthens (by 4.1 cm-1) on saturation of the anti C=C double bond. This is due to the loss in π-σ* donation i.e. there is less eletron density in the σ*C-Cl orbital and hence less antibonding character. The syn C=C bond, however, only strengthens slightly (by 0.6 cm-1) and so the anti C=C bond only affects the syn bond slightly. As the C-Cl bond strengthens, its bond length will shorten slightly (the MOPAC PM6 simulation does not predict a change in bond length). Oddly enough, the model suggests that the chlorine atom is infact closer to the syn C=C bond in the mono-alkene. One might expect the Chlorine atom to be further away from the syn C=C bond in the mono-alkene, thereby reducing its electronegative influence (through space) on the π-cloud, thereby strengthening the C=C double bond. If this "thought-experiment" is infact true, this may reveal a limitation in the modelling technique.
Substituents on the Anti-alkene
Insert ChemDraw diagram of 11a 11b and 11c
| Molecule | C-Cl stretch /cm-1 | syn C=C stretch /cm-1 | anti C=C stretch /cm-1 |
|---|---|---|---|
| 11A | 768.09 | 1756.29 | - |
| 11B | 766.80 | 1756.26 | - |
| 11C | 764.58 | 1757.05 | 1733.03 |
An anti C=C stretch was not assigned for both 11A and 11B. This may be because the double bond no longer exists due to effects from substituents.
Mini Project
This mini-project will be based on a paper by Armstrong et al: Clarification of the Role of Water in Proline-Mediated Aldol Reactions. The project will aim to "check" the data provided by the study and use modelling to characterise isomers of a product.
Reactions of Aromatic Aldehydes with Proline
13C-NMR
The literature states that the NMR spectra were recorded on a Bruker AV400 spectrometer. (8a) and (8b) were identified from various spectra including 13C spectra as the mixture in a ratio of 2.5:1 in d6-DMSO.
(8a):
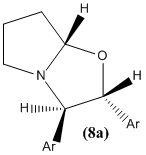
13C-NMR (100 MHz, CDCl3): δ 139.2 (Ar-C), 137.3 (Ar-C), 133.7 (Ar-C), 133.0 (Ar-C), 129.5 (Ar-CH), 129.4 (Ar-CH), 129.1 (Ar-CH), 129.0 (Ar-CH), 128.5 (Ar-CH), 127.7 (Ar-CH), 127.2 (Ar-CH), 127.2 (Ar-CH), 98.9 (C-8), 83.6 (C-2), 74.1 (C-3), 56.0 (C-5), 31.6 (C-7), 23.9 (C-6)
(8b):
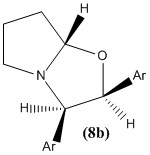
13C-NMR (100 MHz, C6D6): δ 138.0 (Ar-C), 135.7 (Ar-C), 133.9 (Ar-C), 133.8 (Ar-C), 130.5 (Ar-CH), 129.5 (Ar-CH), 129.1 (Ar-CH), 129.0 (Ar-CH), 128.6 (Ar-CH), 126.3 (Ar-CH), 125.9 (Ar-CH), 98.2 (C-8), 76.4 (C-2), 68.7 (C-3), 55.4 (C-5), 32.9 (C-7), 23.9 (C-6). (The missing Ar-CH signal is hidden by the solvent.)
The NMR data is once again in fairly good agreement with the literature.
NOESY
NOESY is a 2-dimensional NMR technique. It can be used to determine whether two protons are spatially close to eachother. For example, in cyclohexane two protons in a 1,3-diaxial relationship will couple in NOESY spectroscopy. However, a proton in an equatorial position will not couple with an axial proton in a 1,3 relationship.
This technique could be used to distinguish between the two isomers 8a and 8b in which two adjacent hydrogens differ in distance from eachother.