Rep:Mod:pp 2
PP_2
Expected: - a title identifying the molecule
- the method and basis set defined
- an image of the summary table produced by gaussview (after optimisation)
- the "Item" table (in pre tags) from the *.log file (from the optimisation job)
- a link to the frequency and/or population analysis files
- low frequencies data (in pre tags) from the *.log file (from the frequency job)
- a rotatable 3d jmol file/image of the optimised structure
- make sure your file link goes to the actual file and not to the wiki page where you saved the file.
What you'll need to be able to report:-
What is the file type?
What is the calculation type?
What is the calculation method?
What is the basis set?
What is the final energy in atomic units (au)?
What is the gradient?
What is the dipole moment?
What is the point group of your molecule?
How long did your calculation take?
Notes
The total energy for any calculation is highly dependent on the quality of the basis set. What this means is that we can NEVER compare the total energy of structures computed using different basis sets (or pseudo-potentials).
You can only compare energies for molecules computed with exactly the same method, AND the same number of atoms each computed with exactly the same basis-set (on every atom)
The method we are using is accurate to ≈ 5 kJ/mol. But because we are comparing similar molecules some of the errors are systematic and so we can assume a better level of accuracy, you should report your numbers to the nearest 1kJ/mol.
Thus we need to report energies in au up to an accuracy of 1 kJ/mol, making the conversion this is 0.0004 au, so report energies in au up to at least 5dp (to avoid rounding errors)
When we carry out a frequency or vibrational analysis we are doing two things at once. The frequency analysis is essentially the second derivative of the potential energy surface, if the frequencies are all positive then we have a minimum, if one of them is negative we have a transition state, and if any more are negative then we have failed to find a critical point and the optimisation has not completed or has failed. The frequency analysis has another important role to play because it provides the IR and Raman modes to compare with experiment.
Demo page for the lab
BH3
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000193 0.000450 YES RMS Force 0.000126 0.000300 YES Maximum Displacement 0.000764 0.001800 YES RMS Displacement 0.000500 0.001200 YES
Frequency file: im_bh3_frequency.log
Low frequencies --- -0.2261 -0.1036 -0.0054 48.0227 49.0824 49.0830 Low frequencies --- 1163.7223 1213.6713 1213.6740
optimised BH3 molecule |
Lowest frequency is outside of the ±15 cm-1 range! Ask for help.
Formally zero frequencies are well separated from the lowest energy positive frequency at 1214cm-1 and the large formally zero frequencies are due to the low level of the basis set and relatively relaxed convergence and integration criteria. This can happen for small molecules. (??)
Vibrational spectrum for BH3
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1164 | 92 | A2 | yes | out-of-plane bend |
| 1214 | 14 | E' | very slight | in-plane bend |
| 1214 | 14 | E' | very slight | in-plane bend |
| 2580 | 0 | A'1 | no | symmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
There are fewer vibrational peaks than vibrations as not all of the vibrations are IR active. To satisfy the selection rules for IR activity, a bend/stretch must result in a change in dipole moment. As the symmetric stretch (2580 cm-1) does not have a change in dipole moment, it is not visible on the spectrum.
PP and basis sets
Frequency file: File:IM BH3 FREQ.LOG
Item Value Threshold Converged? Maximum Force 0.000190 0.000450 YES RMS Force 0.000095 0.000300 YES Maximum Displacement 0.000747 0.001800 YES RMS Displacement 0.000374 0.001200 YES
MOs
Are there any significant differences between the real and LCAO MOs? What does this say about the accuracy and usefulness of qualitative MO theory?
MOs for BH3
| MO (incl. symmetry) | Energy (eV) | Picture | Filled? |
| 1a1' | -6.77 |  |
Y |
| 2a1' | -0.51 |  |
Y |
| 1e' | -0.35 |  |
Y |
| 1e' | -0.35 |  |
Y |
| 1a2 | -0.07 |  |
N |
| 3a1' | 0.17 |  |
N |
| 2e' | 0.18 | 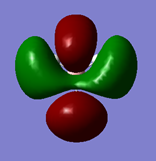 |
N |
Energies
1 a.u. = 627.50 kcal/mol 2625.5 kJ/mol
E(NH3)= -56.55776873 AU // -56.55664155
E(BH3)= -26.61532342 AU
E(NH3BH3)= -83.22468893
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
= -83.22468893 - [-56.55776873-26.61532342] = 0.0515968 au = 135.467398 kJ/mol
Based on your energy calculation is the B-N dative bond weak, medium or strong? What comparison have you made to come to this conclusion?


