Rep:Mod:pmpmpsunzyw
Yawen Zhang 3rd Computational Lab Module one
The basic techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
Molecular Mechanics (MM) method would be used to attempt calculations in this modelling course. This method would allow us to optimise the molecular geometry with minimized energy and analyse the energy contributions, i.e. stretching, bending and torsion.
The Hydrogenation of Cyclopentadiene Dimer
Cyclopentadiene endo dimer 2 is the preferred product comparing with exo dimer 1. By applying the MM2 method on these two molecules, it can be seen that endo dimer 2 is about 3kcal/mol higher in energy. This would lead to the conclusion that the observed reactivity towards cyclodimerisation is due to kinetic factors. So we are considering the transition state stability not product stablity. Although the product 1 has greater stability than product 2, transition state of 2 has greater stability. This observation of reactivity can also be seen from the geometry of two dimers. endo dimer 2 is stablised by the appearance of secondary orbital interaction, whereas it is absent in exo dimer.1
dimer 1
Pentahelicene |
dimer 2
Pentahelicene |
Looking at the relative energies of the hydrogenated dimers,The total energy of dihydro derivative 4 is about 5 kcal/mol less than 3. Derivative 3 has similar energy to dimer 2. According to the thermodynamic prediction of this hydrogenation process, derivative 3 would more likely to form, which means the double bond in the 5-member ring would hydrogenated.
| derivative 3 | derivative 4 | |
|---|---|---|
| Stretch | 1.2266 | 1.0963 |
| Bend | 18.8517 | 14.5074 |
| Torsion | 12.2412 | 12.4972 |
| 1,4 VDW | 5.7571 | 4.5124 |
| Total Energy kcal/mol | 35.9323 | 31.1540 |
derivative 3
Pentahelicene |
derivative 4
Pentahelicene |
By looking at the contribution of energy of derivatives 3 and 4, the energy is mainly contributed from bending and torsion. The main difference beteween 3 and 4 is from bending. 3 requies more energy to bend, so 3 is more rigid than 4. Considering the structures of 3 and 4,4 is relifed from the ring strain, so it must be more stable.
Key literature
- C.S.Wannere, A.Paul, R.Herges, K.N.Houk, H.F.Schefer III, P.Schleyer, J. Comp. Chem., 28, 2006, (1)DOI:10.1002/jcc.20532
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
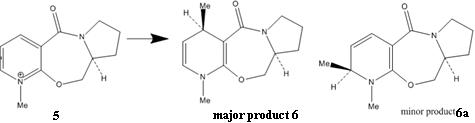
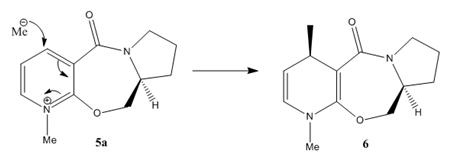
The optical active derivative of prolinol(5) react with Grignard reagents highly regioselectively and stereoslectively to give one major product 6 and one minor product 6(a). The major diastereoisomer 6 has the methyl groups oriented anti to the H atom at the chiral carbon of of the L-prolinol auxiliary. This high regio- and stereoslectivity of this addtion is due to the chelation control. The following mechanism shows how the methyl group is delivered to the geometry shown in the structure. The major diastereoisomer resulting from Grignard reagent MeMgI addition to 5 has the groups Me positioned anti to the H atom at C(4).1
5
Pentahelicene |
6a
Pentahelicene |
| 5 | 6a | |
|---|---|---|
| Stretch | 1.1857 | 1.4711 |
| Bend | 11.3852 | 14.6296 |
| Stretch-Bend | 0.0543 | 0.1653 |
| Torsion | 5.0843 | 5.4479 |
| Non-1,4 VDW | -2.0105 | -2.0418 |
| 1,4 VDW | 11.8934 | 13.2634 |
| Charge/Dipole | 2.6985 | |
| Dipole/Dipole | -3.9689 | -4.0661 |
| Total Energy kcal/mol | 26.3219 | 28.8694 |
The energies of 5 and 6a are mainly built upon bending and torsion. By looking at the structure of 5, the carbonyl group is slightly out of the plane with respect to the aromatic ring. After alternating the position of one atom each time and running MM2 for several times, the carbonyl group shows same orientation with respect to the aromatic ring. The carbonyl group is confirmed that it is slight bent out of the plane.
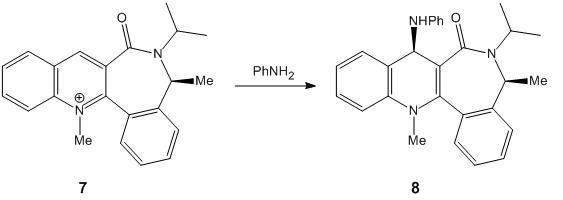
In the second example, the pyridinium ring of 7 reacts with aniline to form 8 by transferring the NHPhenyl group to electrophiles.
| 7 | 8 | |
|---|---|---|
| Stretch | 1.5972 | 1.3872 |
| Bend | 6.7991 | 13.3042 |
| Stretch-Bend | 0.3157 | 0.2289 |
| Torsion | -6.1004 | -3.7938 |
| Non-1,4 VDW | -1.6787 | -3.0234 |
| 1,4 VDW | 17.5783 | 14.4345 |
| Charge/Dipole | 2.3176 | |
| Dipole/Dipole | -4.8179 | -5.4393 |
| Total Energy kcal/mol | 16.0110 | 18.9063 |
7 is a axially chiral quinolinium salt. In this case, chiral quinolinium salt is reacted as an 'amide transferring agent'.This reaction is an application of atropenantioselective amidification. The chiral masked amides, with a chiral axis C3-C=O, is the main configurational element respond to the highly diastereslective 1,4 reduction of chiral quinolinium salt. Consiering the steric hinderce, attacking group NHPh must orient anti to the carbonyl group.2 This consquence can be seen from the Jmol structure of 8.
7
Pentahelicene |
8
Pentahelicene |
In 7, the carbonyl group points out of the plane to a larger angle with respect to two aromatic rings (one aromatic ring contains N and the 6-carbon ring next to it).
MM2 calculation is less useful in this modelling case,as the the reaction is 'kinetic control'. Transition state stability is more important than product stability. A better method which can predict the transition state would provide more accurate information of this reaction.
Key literature
- A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- Leleu, Stephane; Papamicael, Cyril; Marsais, Francis; Dupas, Georges; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928. DOI:10.1016/j.tetasy.2004.11.004
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
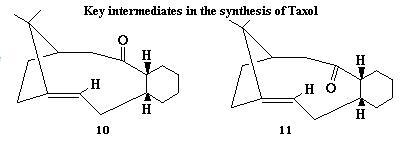
In the synthesis of Taxol,there is a key intermedia,it can be either 10 or 11 with the carbonyl group pointing up or down.MM2 calculation is applied to decided which isomer is more stable.
| 10 | 11 | |
|---|---|---|
| Stretch | 2.3440 | 2.6006 |
| Bend | 12.6810 | 15.5737 |
| Stretch-Bend | 0.4765 | 0.3347 |
| Torsion | 15.2082 | 16.0344 |
| Non-1,4 VDW | -2.0429 | -0.7207 |
| 1,4 VDW | 12.0686 | 12.5987 |
| Dipole/Dipole | 0.2401 | 0.2638 |
| Total Energy kcal/mol | 41.0115 | 46.6852 |
The stereochemistry of carbonyl addition depends on which isomer is more stable. Intermediate 10 has the carbonyl group pointing up as well as the bridged H atoms, whereas the intermedia 11 has it pointing down.The structures of these two intermediates are altered slightly to achieve the lowest energy as possible. It is found that intermedia 10 is about 5kcal/mol lower in energy. The main energy contribution is from bending, torsion and 1,4-VDW.
Intermediate 10
Pentahelicene |
Intermediate 11
Pentahelicene |
During subsequent functionalisation of the alkene, the intermediate reacts very slowly due to the appearance of hyperstable alkene which are less strained than the parent hydrocarbon. The hyperstable alkene shows reduced reactivity due to the bridgehead location of the double bond. Generally speaking, this extreme stability of this kind alkene is afforded by the cage strcture of the alkene and to the greater strain of the parent polycycloalkane.1
Key literature
- W. Maier, P. Von Rague Schleyer. J. Am. Chem. Soc., 1981, 103(8), pp 1891-1900DOI:10.1021/ja00398a003
How one might induce room temperature hydrolysis of a peptide
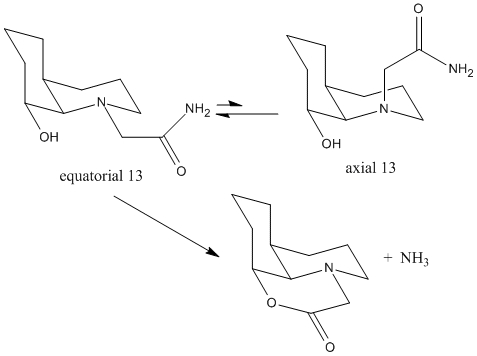
Kinetic behaviour can be simply rationalized by conformational analysis. By acceleration of some enzymes, the hydrolysis of a peptide bond can be achieved within one second rather than 500 years. The two isomers 13 and 14 with only different stereochemistry shown below can accelaration the hydrolysis by different rates.
For cis-isomer 13, the N-substituents can orientate to the axial or equatorial position with respect to the decalin ring. The energies of both forms can be calculated by MM2 calculation.
axial 13
|
equatorial 13
| |||||||
|---|---|---|---|---|---|---|---|---|
| Stretch | 1.4358 | 1.9927 | ||||||
| Bend | 7.8368 | 5.7186 | ||||||
| Stretch-Bend | 0.5939 | 0.6092 | ||||||
| Torsion | 11.5090 | 9.5686 | ||||||
| Non-1,4 VDW | -8.2070 | -5.7339 | ||||||
| 1,4 VDW | 10.4778 | 10.0426 | ||||||
| Dipole/Dipole | -4.3341 | -4.5859 | ||||||
| Total Energy kcal/mol | 19.3124 | 17.6119 |
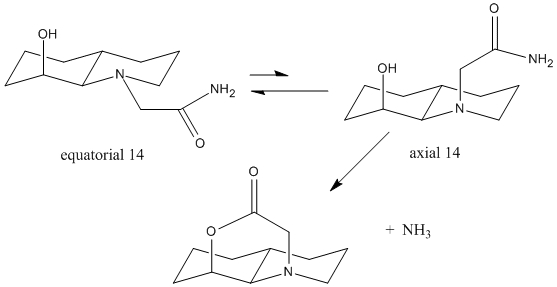
For trans-isomer 13, the N-substituents can also orientate to the axial or equatorial position with respect to the decalin ring.
axial 14
|
equatorial 14
| |||||||
|---|---|---|---|---|---|---|---|---|
| Stretch | 1.6895 | 1.4602 | ||||||
| Bend | 5.4566 | 3.9300 | ||||||
| Stretch-Bend | 0.6061 | 0.5049 | ||||||
| Torsion | 8.7654 | 7.7079 | ||||||
| Non-1,4 VDW | -5.1270 | -7.1687 | ||||||
| 1,4 VDW | 9.5248 | 9.9119 | ||||||
| Dipole/Dipole | -6.8358 | -6.6146 | ||||||
| Total Energy kcal/mol | 14.0797 | 9.7317 |
By considering both axial and equatorial forms of stereoisomers 13 and 14, the tran-isomer 14 with the ethylamido ute at the equatorial positon is achieved with the lowest energy. Overall, ethylamido groups prefers to be in the equatorial position with lower energy.
The reaction is accelerated due to pseudo A 1,3 strain between the pypyrrolidine ring and adjacent methyl group. The intramolecular reaction relives the steric starin to achieve the huge increase in rate.
In order this hydrolysis reaction to happen, the OH group must orient close enough to the ethylamido group, and at the right angle of approach. In the energetic preferred equatorial iosmer 13, ethylamiso group can undergo intramolecuar nuleophilic attack by the hydroxyl group. But it is different case for isomer 14. Equatorial 14 must convert to axial 14 first, in order to achieve the right orientation. This explains why isomer 4 reacts 400 times quicker than 5.1
Key literature
- M. Fernandes, F. Fache, M. Rosen, P.-L. Nguyen, and D. E. Hansen, 'Rapid Cleavage of Unactivated, Unstrained Amide Bonds at Neutral pH', J. Org. Chem., 2008, 73, 6413–6416 ASAP: DOI:10.1021/jo800706y
Modelling Using Semi-empirical Molecular Orbital Theory
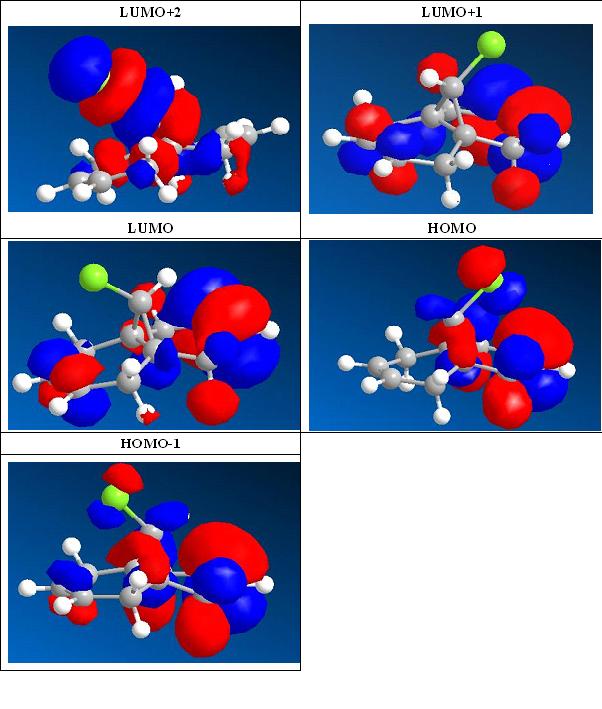
In this exercise, two different methods were used to predict the geometry of compound 12 and its hydrogenated version 12-1. We start from a classical mechanical method MM2 calculation followed by HF/STO-3G self-consistent-field MO method. This would provide an approximate representation of the valence-electron molecular wavefunction. By optimizing different molecular orbitals of 12, the following chart is obtained.
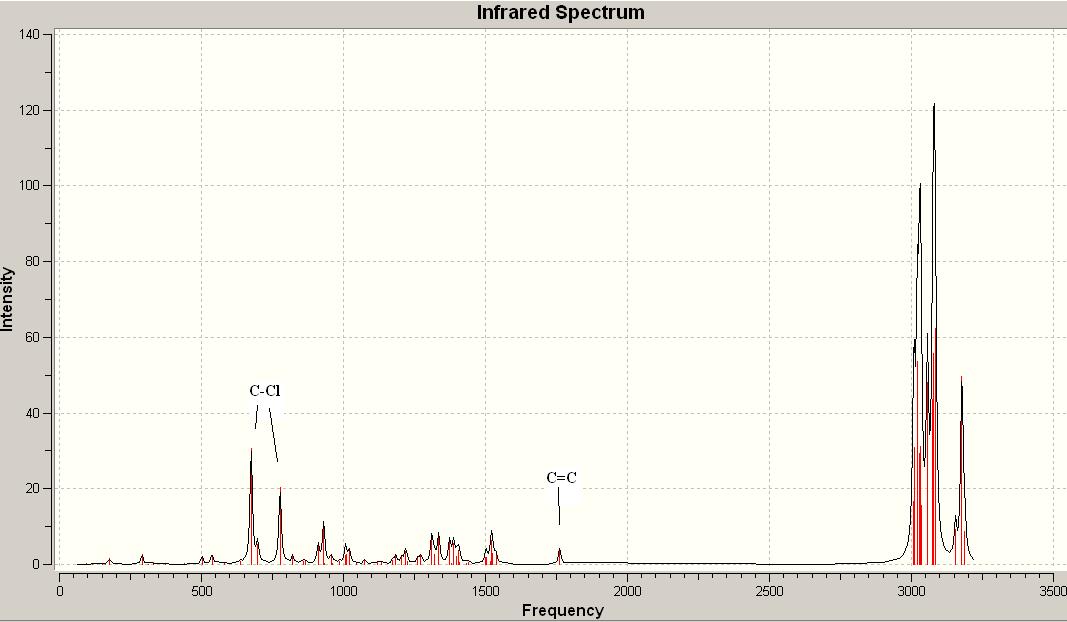
Then a more accurate Density-functional B3LYP/6-31G(d) approach was used to obtain the wave-description of the electrons. Analysing the IR spectra obtained from this method, particularly pay attention to C-Cl stretch and C=C, we can see that the C-Cl stretches show more higher intensity than C=C stretch. As predition from the structure itself, 12 shows two peaks of C=C stretch, whereas 12-1 shows only one due to shorten of one C=C bond.
12 reacts with electrophilies regiospecifically on the double bond endo to the Cl substituent, on the face anti to the C-Cl. This regioselection can either determined by orbital or electrostatic control or specific factor present only in transition state. In 12, antiperiplanar overlap with C-Cl σ* (LUMO+2) orbital gives stablised exo π-orbital (HOMO-1). Instead, 12-1 only contains one C=C bond, shorten for one π-orbital. This may explain why the C-Cl stretches in 12 have much stronger intensities.1
12
|
12-1
| |||||||
|---|---|---|---|---|---|---|---|---|
| C-Cl stretch | 690.448(54.9928)
772.634(25.2418) |
675.084(30.5215)
776.849(20.2345) | ||||||
| C=C stretch | 1740.76(4.1445)
1760.91(3.9036) |
1761.69(4.3051) |
IR spectrum of 12

Key literature
1. B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
2. :DOI:10042/to-1736
3. :DOI:10042/to-1737
Structure based Mini project using DFT-based Molecular orbital methods
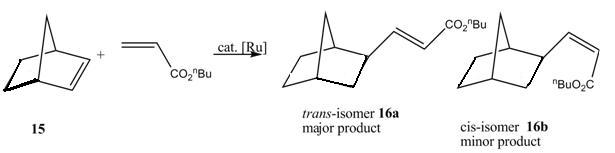
Ruthenium-Complex-Catalyzed Regio- and Stereoselective Linear Codimerization of 2-Norbornens with Acrylic Compounds
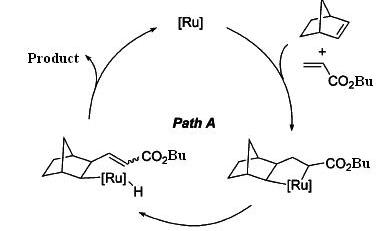
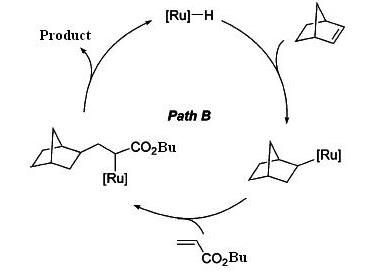
A linear codimerization of 2-Norbornens with acrylic compounds catalyzed by ruthenium complex efficiently, gives exo-trans-2-norbornylacrylates as major products and cis-isomer as minor products.6
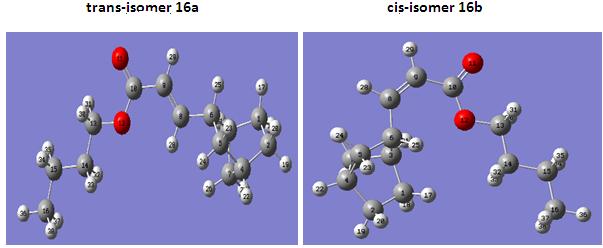
trans-isomer 16a
Pentahelicene |
cis-isomer 16b
Pentahelicene |
There are two possible reaction schemes for this linear codimerization. In path A, 2-norbornene and acrylate bind to ruthenium active species first, and followed by reductive elmination to give products. In path B, ruthenium hydrates first, and 2-norbornene insert into Ru-H bond, followed by further insertion of acrylate into Ru-C. After β-elmination, the products are released6. By MM2 calculation of both isomers, it is found that the trans-isomer is slightly lower in energy, so it is energetic favoured. However, they only differ in about 1kcal/mol. If we just simply look at the structures of both isomers, cis-isomer could be disfavoured due to steric hinderence. The long chain nBu group would avoid interaction with the 6-membered ring, therefore it would require more energy to stablise and be less favoured.
The stereochemistry of 16a and 16b can be determined by looking to the observation of NOE in their 1H NMR spectra. In cis-isomer 16b, NOE interaction is observed between the protons attached to the allylic carbons, whereas the NOE interaction is not observed in trans-isomer 16a. Result in the NMR data, trans-isomer would normally give a 15Hz 3JH-H between the protons attached to the C=C bond and cis-isomer would give 10Hz 3JH-H instead. This prediction is confirmed by the reference (15.7Hz and 11.7Hz respectively).6
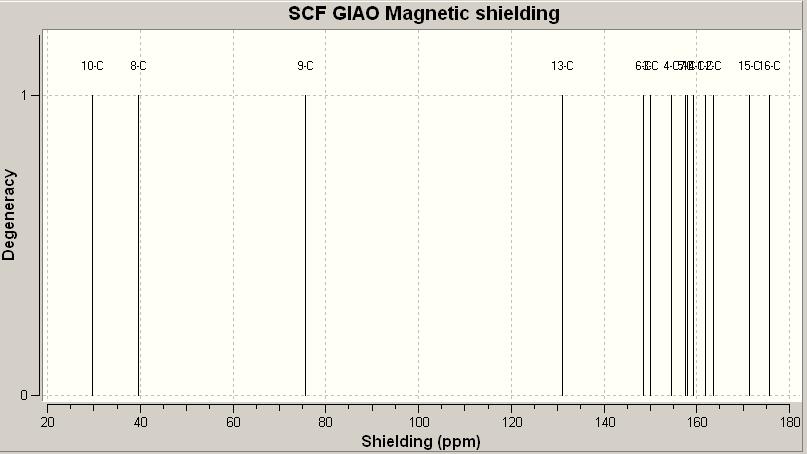
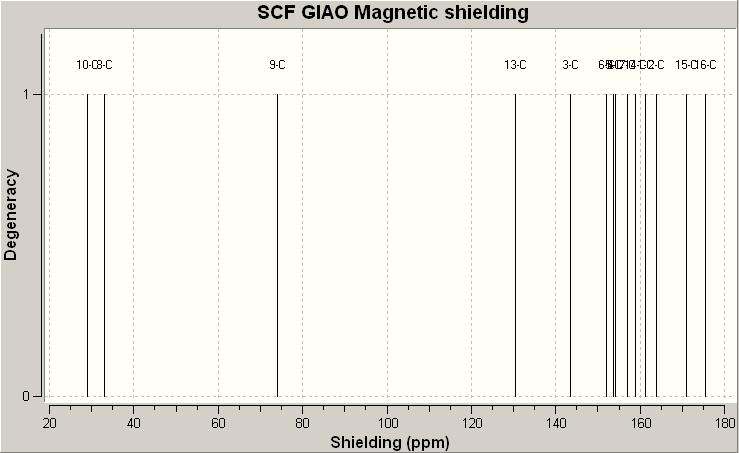
The 13C NMR spectra of both isomers are predicted by GIAO approach, and it gives the following results.
13C NMR spectrum of trans-isomer 16a
13C NMR spectrum of cis-isomer 16b
After applying the correction for carbonyl carbon of esters (yellow cells) by the formula δcorr = 0.96δcalc + 12.2, calculated chemical shifts can be used for analysis. Comparing the obtained chemical shifts from GIAO approach with the reference values, there is hugh difference between them. This may due to the orientation of the molecule. Both molecules contain 6-membered ring, chair conformation always has lower energy than boat conformation. But in this case the 6-membered ring with bridged methylene group would not give a chair conformation after applying MM2 calcuation. Also the 4 carbon chain attach to the ester is quite flexible, it can twist with varies angles. Both these factors would lead to a unaccurate geometry structure as a starting point of predicting NMR using GIAO approach.
table of comparing the GIAO calculated data and refernce data 6
Optical rotaion is also calculated by the method b3lyp/aug-cc-pvdz in chlroform. trans-isomer 16a is found as [Alpha]D= 62.14 deg and cis-isomer 16b is found as [Alpha]D=-228.85 deg. Both of them give reliable values, with magnitude greater than 50 deg. The clear difference of these two values of OR in sign suggests the stereochemistry between these two molecules. This apporach is working successfully.
Overall, the computaional methods of predicting chemical data and modelling are working reasonablly well and can be used for further analysis. If better NMR data is needed, the method which is less geometry sensitive should be used instead.
Key Literature
1. trans-isomer optimization:DOI:10042/to-1698
2. cis-isomer optimization::DOI:10042/to-1697
3. trans-isomer NMR:DOI:10042/to-1699
4. cis-isomer NMR:DOI:10042/to-1700
5. cis-isomer OR:DOI:10042/to-1701
6. Y.Ura, H.Tsujita, K.Wada, T Kondo, T Mitsudo, J. Org. Chem., 2005 70 (17)DOI:10.1021/jo050413o
7. trans-isomer OR:DOI:10042/to-1739