Rep:Mod:pks1927project
Mini Project
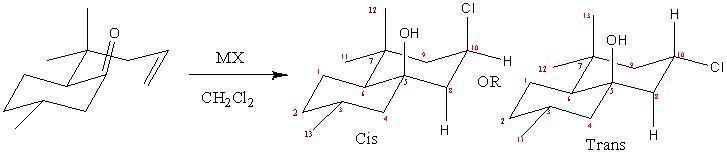
The Prins reaction is an electrophilic addition of a ketone or an aldehyde to an alkene or alkyne. This reaction yields various products depending on the reaction conditions, for example, 1,3 diols, ester, dioxane or allyl alcohols can be formed. There are many variations to the Prins reaction because the reaction can undergo cyclisation and the oxo-carbenium ion that is formed can react with nucleophiles if present in the reaction mixture.
Unsaturated aldehyde and ketones in the presence of Lewis acids can undergo an intermolecular Prins reaction. These reactions are very useful as they are able to form heterocycles, which are present in many drug molecules. The stereochemistry of a Prins reaction is dependent on the electrophilic addition across the carbon double bond. If syn addition occurs then a cis product forms and if an anti addition occurs a trans product forms. Prins cyclisations tend to be anti stereoselective and hence the trans isomers predominate, but when certain Lewis acids are used in this reaction syn addition is preferred, which results in the cis isomer to predominantly form. The reason for the change in stereoselectivity is due to neighbouring group participation and the formation of a cyclic and lower energy transition state.
MX in the diagram represents the Lewis acid used. If MX is SnCl4 then the ratio of cis to trans isomer formed is 1:50, but if the Lewis acid is TiCl4 then the ratio will be 10:1. The energies of the cis and trans isomers where found by using MMFF94 force fields (see table). The trans isomer is more stable and hence the one more likely to form. This is expected because in the cis isomer the Cl and OH are both in axial positions on the cyclohexane ring and hence the cis isomer will experience strain due to 1,3 diaxial compressions. The trans isomer does not have this strain energy and hence is more stable (lower in energy). The trans isomer is also preferred as it has a lower overall dipole compared to the cis isomer. The reason why the TiCl4 Lewis acid promotes the formation of the cis isomer is due to the mechanism below.
| Molecule | Energy/ kcalmol-1 | Energy/ kJmol-1 |
|---|---|---|
| Cis isomer | 43.74 | 183.01 |
| Trans isomer | 39.43 | 164.98 |
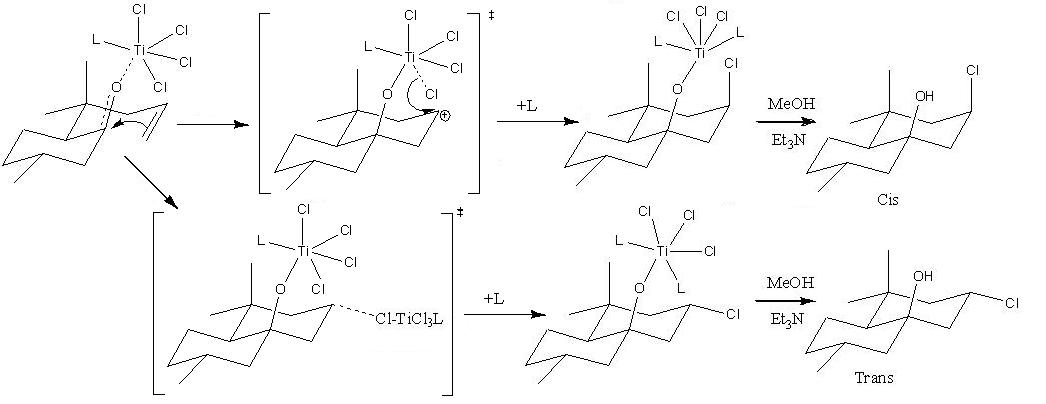
The mechanism shows that the TiCl4 weakly interacts with the carbonyl oxygen in the unsaturated aldehyde and hence the incoming direction of the Cl atom is fixed to be axial and hence the cis isomer forms more easily. This neighbouring group participation does not occur when SnCl4 is used and hence the cis product does not predominate as the more thermodynamically stable isomer forms (trans). Another reason why the cis isomer is predominantly formed when TiCl4 is used in a Prins cyclisation reaction is because the energy of the transition state to form the cis isomer is lower than the energy of the transition state, which forms the trans isomer. This is because to form the cis isomer the Cl atom is provided intramolecularly, but to form the trans isomer another molecule of TiCl4 is needed to provide the Cl atom (intermolecularly). Finally, the cis isomer predominates when TiCl4 is used because an excess of TiCl4 is needed to form the trans isomer (see mechanism).
Characterisation of the Cis and Trans Isomers
To determine the difference between the isomers and hence identify which one has formed, various techniques can be used. Both proton and carbon NMR spectroscopy can be used because even though the isomers have the same atoms in similar environments, the chemical shifts produced by each isomer will be slightly different (this is shown below). The proton or carbon chemical shifts in an NMR spectrum for each isomer do not change drastically and hence the there is room for error when using NMR spectra to differentiate between isomers.
A major fact which helps distinguish between the isomers is the 3JHH coupling constants. This is because if protons are three bonds away from one and other and they are trans to one and other the coupling constant will be larger than if the protons were cis to one and other (see below). Another way to distinguish between the two isomers is by determining the optical rotation of the product. The optical rotation of the cis isomer is different to the trans isomer. The literature value for the optical rotation of the cis isomer above is + 2.7o and for the trans isomer it is +5.7o.
Other spectroscopic methods can be used to analyse the isomers to see what bonds are present and what atoms are present in the molecule, but they will not differentiate between the two isomers. For example, IR, mass spectroscopy and UV-Vis spectrometry all provide information about the molecule, but the same information will be produced for both isomers as the same number of atoms and same bonds are present in both isomers.
Carbon NMR
The following predicted Carbon NMR spectra were obtained by minimising the energy of each isomer with the MM2 force field and then by using the method DFT=mpw1pw91 and the basis set 6-31G(d,p) an input file was created and sent to scan. Once the geometry had been optimised by scan the output file was renamed and the code was changed, this modified file was sent to the scan again to obtain an NMR data file. The NMR reference used was TMSmPW1PW91/631(d,p)CDCl3GIAO.
| Cis Isomer | |||
|---|---|---|---|
| Carbon Atom Nos | Predicted Shifts/ppm | Literature Vlaues/ppm | Difference/ppm |
| 5 | 70.2461 | 72.0 | -1.7539 |
| 10 | 60.7568 | 57.3 | 3.4568 |
| 6 | 50.7913 | 50.9 | -0.1087 |
| 4 | 50.2059 | 50.8 | -0.5941 |
| 9 | 46.6251 | 47.4 | -0.7749 |
| 8 | 44.7403 | 46.2 | -1.4597 |
| 2 | 35.5863 | 36.0 | -0.4137 |
| 7 | 33.7539 | 33.3 | 0.4539 |
| 11 | 33.1371 | 32.9 | 0.2371 |
| 3 | 28.1942 | 27.4 | 0.7942 |
| 12 | 24.993 | 24.4 | 0.7942 |
| 1 | 24.0522 | 22.5 | 1.5522 |
| 13 | 23.1461 | 22.0 | 1.1461 |
| Trans Isomer | |||
|---|---|---|---|
| Carbon Atom Nos | Predicted Shifts/ppm | Literature Vlaues/ppm | Difference/ppm |
| 5 | 73.5146 | 73.8 | -0.2854 |
| 10 | 58.0224 | 54.6 | 3.4224 |
| 8 | 51.8881 | 52.4 | -.5119 |
| 9 | 51.4224 | 51.0 | 0.4224 |
| 6 | 50.2115 | 50.5 | -0.2885 |
| 4 | 50.0982 | 50.0 | 0.0982 |
| 7 | 37.7153 | 35.7 | 2.0153 |
| 2 | 35.5167 | 35.3 | 0.2167 |
| 12 | 32.0915 | 31.7 | 0.3915 |
| 3 | 28.8773 | 27.6 | 1.2773 |
| 1 | 23.807 | 22.1 | 1.707 |
| 13 | 23.4426 | 22.0 | 1.4426 |
| 11 | 22.8042 | 21.4 | 1.4042 |
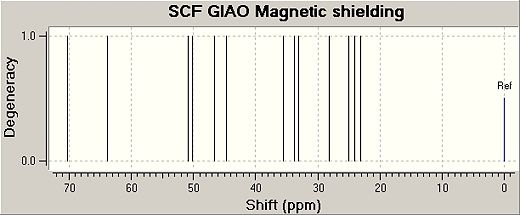
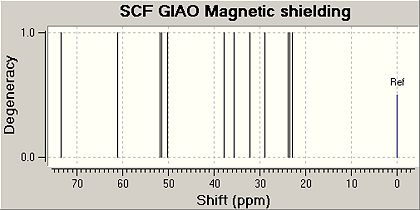
The data obtained for both isomers are very close to the literature values and hence the GIAO method can predict carbon NMR data accurately. The reasons why the method worked could also be because a rigid and fairly small molecule was analysed. Errors may occur when more flexible and large molecules are analysed.
The carbon NMR data (predicted and experimental) for the cis isomer gives different chemical shifts for carbon atoms 8, 9 and 10 compared to the trans carbon NMR data (predicted and experimental). These carbon atoms cause the NMR spectra for both isomers to be different from one and other and hence help indicate if the correct isomer has been identified. It should be noted that the carbons labelled 11, 12 and 13 on the cis isomer are not in the same position as the carbon atoms labelled 11, 12 and 13 on the trans isomer. The number 11 carbon atom on the cis isomer is carbon 12 on the trans isomer, 12 on the cis isomer corresponds to 13 on the trans isomer and 13 on the cis isomer is equivalent to 11 on the trans isomer. This assignment was done by the computer.
Predicting Coupling Constants
The coupling constants between trans hydrogen atoms that are three bonds away are larger than coupling constants for hydrogen atoms cis to one and other. The coupling constant were found for the hydrogen atoms indicated in the cis and trans isomers diagram above. They were found by using the NMR data generated by the scan and converting the file into a .mol and uploading it onto the Janocchio website. This site depicted the coupling constants between any protons that were selected, by using the dihedral angle between the protons and the Karplus equation. The coupling constant between the two hydrogens in the cis isomer was 2.9203Hz and the coupling constant for the hydrogen atoms in the trans isomer was 11.5695Hz, which verifies that the trans protons give bigger coupling constants.
The literature values for the cis coupling constant is 3.6Hz and for the trans coupling constant it is 12.6Hz, these values are fairly close to the predicted values and hence convey that the literature values are correct and that the NMR data produced for each isomer is fairly accurate. In addition, the accuracy of the predicted data shows that the optimised geometries obtained for both isomers from the various methods described above are very good as the molecule produced has yielded good results.
IR Spectra
The molecules were minimised by using the MM2 force field and the MOPAC interface (PM6 method). Then an input file was created and sent to scan with the appropriate code to obtain IR data. The results obtained are shown below:
| Cis Isomer | ||
|---|---|---|
| Bond | Predicted Frequency/cm-1 | Literature Vlaues/cm-1 |
| O-H Stretch | 3733.18 | 3592 |
| C-H Stretch | 3184.51 | 3479 |
| C-H Stretch | 3004.73 | 2947 |
| C-H Stretch | 2973.85 | 2867 |
| C-H Scissoring and Bend | 1459.13 | 1455 |
| C-H Scissoring and Bend | 1376.70 | 1370 |
| C-H Twist | 1255.56 | 1265 |
| C-C Scissoring | 1167.48 | 1167 |
| C-O Stretch | 1046.37 | 1009 |
| C-Cl Stretch | 576.71 | - |
| Trans Isomer | ||
|---|---|---|
| Bond | Predicted Frequency/cm-1 | Literature Vlaues/cm-1 |
| O-H Stretch | 3720.77 | 3567 |
| C-H Stretch | 3163.00 | 3483 |
| C-H Stretch | 3004.36 | 2948 |
| C-H Stretch | 2994.12 | 2869 |
| C-H Scissoring and Bend | 1455.13 | 1456 |
| C-H Scissoring and Bend | 1374.38 | 1368 |
| C-H Twist | 1241.57 | 1240 |
| C-O Stretch | 1049.97 | 1028 |
| C-Cl Stretch | 778.47 | 775 |
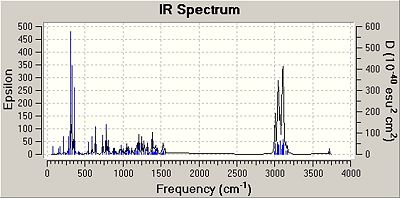
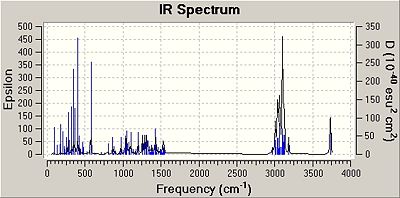
The predicted IR frequencies do not fit well with the literature value for both isomers at higher wavenumbers. The OH bond stretch frequencies for both isomers are out by about 300 cm-1. However, for frequencies around 1000 cm-1 the predicted and the literature values match fairly well. This is because the IR data recorded was done using a solution, but the IR data predicted is based on gaseous molecules and hence the solvent interactions with the molecules are not taken into account by computational methods. At higher frequencies the error is substantial but at lower frequencies the error is much less noticeable.
The IR data predicted has many more frequencies than recorded, which could be due to the fact that the IR data predicted is done by assuming the molecules are in the gaseous state and hence show more frequencies as gaseous molecules do not interact with solvent molecules. Whereas the literature IR spectrum may have less peaks due to solvent interactions which may prevent certain vibrations to be shown.
In addition, the IR data in the literature did not report a C-Cl stretch for the cis isomer, which is essential in verifying that the correct compound has been made. The reasoning for this is may be that the stretch is in the fingerprint region and may not have been seen clearly. However, the predicted data did have a value for this stretch and this value seems to be correct as it is within the region that a C-Cl bond stretch frequency should appear (600-800cm-1).
Optical Rotations
An attempt was made to predict the optical rotations of each isomer as the literature reported these values and the values are significantly different between the two isomers and hence should be used to distinguish between the isomers. However, no data was obtained as the calculations took too long to run. The scan stopped running the calculation after 48 hours. Even if values were obtained for the optical rotations of both isomers, it is likely that the values would have been inaccurate and very different from the literature values. This is because any optical rotation values that are less than 100o will yield unreliable values from computational analysis. The literature value for the optical rotation of the cis isomer is + 2.7o and for the trans isomer it is +5.7o.
Conclusions
Overall the computational analysis on the above isomers was a success because the results obtained were very close to the literature values. The predicted NMR data obtained was more accurate and close to the lit values compared to the predicted IR data. However, it is established that the reason for the slight discrepancies between the predicted and experimental IR data values is due to the fact that computational methods predict IR data for gaseous molecules. The IR data in the literature is produced form isomers in solution and hence will have solvent interactions occurring, which changes the vibrational values obtained. If the literature had IR data, which was run on the isomers in the gaseous state I am sure that the literature data would match the predicted IR data.
Even though computational analysis proved to be useful and was able to predict correctly the NMR and IR data for both isomers, there still are some problems with computational analysis, as errors in predicted data do occur if the molecule is flexible or too large. Also unfortunately molecules with heavy atoms cannot be analysed computationally, which limits the molecules that can be analysed.
However, overall computational analysis is proven to be useful as it can help scientists to analyse, predict and assign NMR and IR spectra and predict which isomer is the more stable one. For example, the literature that has been used in this project had carbon NMR and IR data for both isomers, but the peaks were not assigned. Therefore from my analysis I have been able to assign the peak values.
Key Literature
R. B. Miles, C. E. Davis, J. Org. Chem., 2006, Vol. 71, No. 4, 1493-1501. DOI:10.1021/jo052142n
