Rep:Mod:physical2124
The Cope Rearrangement Tutorial
Within the Cope Rearrangement there are two different possible transition states; there is the boat form and the chair form. Using Gaussview, and Gaussian it is possible to predict the energies of both of these transition states, as well as being about to predict what they look like. Initially a molecule of 1,5-hexadiene was drawn out with an 'anti' linkage. Using the method of Hartree Fock and the base set of 3-21G the molecule was optimised to give an energy of -231.69 AU. The symmetry of the molecule was given as Ci, and the resulting optimisation can be seen in figure 1.


I would expect a moleucle of 1,5-hexadiene with the gauche linkage to have around the same energy of that with an anti linkage as both the transition states are of equal energy, therefore it would make no difference to the total energy if the orientation of the initial molecule were to be different. This was tested by drawing out a 1,5-hexadiene molecule with a gauch linkage (figure 2). The same method and basis set were used as before, and the optimised structure (figure 3) was returned with an energy of -231.68 AU. As you can see the energy difference between the two conformers is minimal, as expected. The point group of the molecule was shown to be C1.


When comparing the structures drawn to those in the table on the module page (INSERT LINK) it can be seen that the first structure drawn is the molecule labelled anti2, and the second molecule drawn in gauche6. This is because they have the same energy, and point groups as those in the table. As the initial molcule drawn in figure 1 had the Ci symmetry the energy of this was compared to that in the table: the energy that was given when the molcule was optimised was exactly the same as that given in the table. Using a different method and base set (B3LYP/6-31G*) the molecule was reoptimised; this is a better base set and is more advanced. The energty of the resulting molecule was given as -234.61 AU. The difference in geometry is not that significant, however second molecule has a longer double carbon bond length, as well as a bigger angle, this would lead to a drop in energy, which is shown.
Using the optimised molecule a frequency calculation was run. This showed that there were no imaginary vibrations, and also enabled me to see the different energies of the molecule (ie electronic, thermal, enthalpy and free energy). The value of these is shown in the table below:
| Type of energy | Energy (AU) |
|---|---|
| Sum of electronic and zero point | -234.47 |
| sum of electronic and thermal energies | -234.47 |
| sum of electronic and thermal enthalpy | -234.46 |
| sum of electronic and thermal free energy | -234.50 |
Optimizing the "Chair" and "Boat" Transition Structures
An allyl fragment was drawn and optimised using the HF/3-21G level; the resulting structure can be seen in figure 6. This fragment was then used to optimise the transition state using two different methods, both of which are outlined below.

The first method was to optimise the structure to a transition state using a TS(Berny) method. This was also used to get a frequency calculation with an IR spectrum. This showed that there was a vibration present at -818cm-1 which corresponds to the making of the bond within the transition state. The vibrational directions can be seen in figure 7. the resulting transition state can be seen in figure 8, which corresponds to that of one shown in the instructions, which also has a bond length of 2.2 Angstroms.

The other method was to use something called the 'Redundant Coordinate Editor'. This essentially locked the length of the bonds and found the transition state with the bonds already set at a certain length. The redundant coordinates were set again to optimise the fixed bond distances. This resulted in the molecule shown in figure 6. The two molecules produced from the two different methods were then compared. From inspection of the distance between the two carbons that either form or break bonds, with the redundant co-ordinate method the distance is 2.2 Angstroms, whereas with the other method the distance is 2.02 Angstroms. This shows that the setting of the distance is better, as you can control it yourself; obviously this is only possible if you know what the optimum distance would be in the transition state.
The Diels Alder Cycloaddition
Using the methods that had already been outlined in this report, I was then able to go on and find the transition states for the reaction of cisbutadiene and ethylene and then the reaction between maleic anhydride and Cyclohexa-1,3-diene, which is based upon the first reaction.
Cisbutadiene
The molecule shown in figure 7 was drawn out in Gaussview and underwent an optimisation calculation. This also produced the HOMO and LUMO for the cisbutadiene molecule. These are shown to the sides:


With regards to the symmetry of both the HOMO and LUMO to the plane, the HOMO is antisymmetric and the LUMO is symmetric.
The transition structure was then determined using the redundant co-ordinate method outlined above, and the resulting molecule can be seen to the right captioned 'Transition State'

The HOMO and LUMO were again plotted and are to the left and the right.
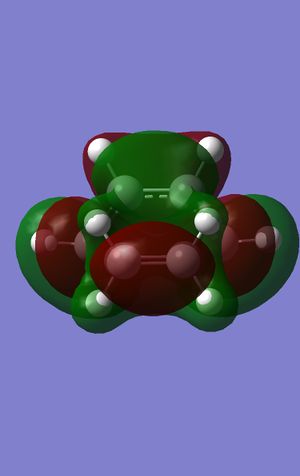
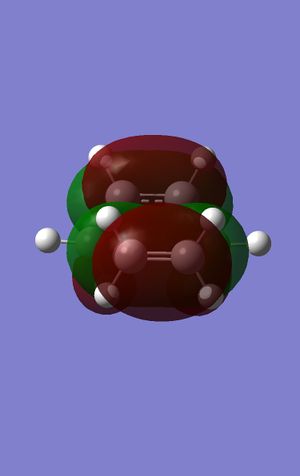
This time the HOMO and the LUMO are both symmetric with regards to the plane. The bond lengths of the carbon-carbon bond breaking and bond forming in transition structure are both 2.21 Angstroms, which corresponds to the general carbon carbon length of a transition state. The frequency calculation showed that there was a vibration present at -885cm-1 which shows the vibration of the bond forming, which is correct as well as being sychronous as they are vibrating in time with each other, i.e. they both go toward each other at the same time, therefore enabling the bond to form. the lowest frequency absorbed (that isn't imaginary) is found at 269cm-1; this is a lot greater than the imaginary vibration. A typical sp3 bond length for a carbon carbon bond is 1.54 Angstrom and for an sp2 carbon carbon bond is 1.38 Angstrom. The Van der Waals radius for a carbon atom is 1.70 Angstrom. This shows that the C-C bond length in the transition state is heading towards an sp3 bond, as it is possibly too long to form an sp2 bond, as well as the Van der Waals radius being greater than that of the sp2 bond length. The HOMO at the transition structure can be seen as being symmetric with respect to the plane.
The overall reaction is allowed due to the symmetry overall being preserved. The MO's that were used to form the HOMO are the LUMO of the butadiene (which is shown above) and the HOMO of the ethylene molecule.
Reaction of Cyclohexa-1,3-diene with Maleic Acid
The endo and exo transition structures were drawn out in Gaussview, and subjected to calculations to optimise them. They were optimised in using the Redundant co-ordinate method again, as outlined before. These optimised structures can be seen to the left and right. The energies of the structures are shown in the table below:


| Transition structure form | Energy (AU) | Bond Length (Å) | Orientation | HOMO |
|---|---|---|---|---|
| Endo | -605.7 | 1.55 | 2.53 | |
| Exo | -605.6 | 2.23 | 2.77 |
The overlap between the (C=0)-O-(C=O) shows that there is more interaction of the Carbons in the endo transition state than there is in the exo transition state; therefore with the secondary orbital overlap in play, the product will prefer to take the endo form, as this will have more interactions and ultimately be lower in energy. The exo form will be more strained due to interactions between the hydrogen and the oxygen, as both the groups are in the same orientation, leading to the interactions, therfore straining the molecule, and meaning it will be higher in energy. The HOMO for both the exo and endo form can be seen below:


References
1.http://www.iop.org/EJ/article/0022-3719/19/24/006/jcv19i24p4613.pdf?request-id=d878cddd-9df8-4f4a-ae69-e5ae8b20808c2.
2.( Bondi, A. (1964). "Van der Waals Volumes and Radii". J. Phys. Chem. 68 (3): 441–51. doi:10.1021/j100785a00)
3. Marye Anne Fox,* Raul Cardona, and N. J. Kiwiet, J. Org. Chem. 1987,52, 1469-1474
