Rep:Mod:phys3TLK2739
Cope rearrangement reaction of 1,5-hexadiene
The Cope rearrangement is an example of a [3,3] sigmatropic reaction. As it is a pericyclic reaction it takes place through a concerted mechanism. The goal of this exercise was to determine the structure of the transition state and to calculate some key parameters of the reaction. The calculations were first performed in the HF/3-21G level of theory and then in a higher B3LYP/6-31G* level, in order to obtain more accurate values of the activation energy[1].
Optimising the reactants and products
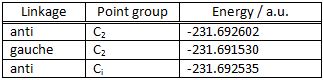
1,5-hexadiene was optimised in the HF/3-21G level of theory so that its most stable conformation could be determined. This was done by drawing the molecule at different conformations, first with an anti linkage of the central four carbons and then with a gauche linkage and optimising to the energy minimum. Ten possible conformations for 1,2-hexadiene have been reported in literature[2]. In this exercise three were located and are shown in the table below. The conformation that has an anti-linkage of the 4 central carbons and a Ci geometry is the lowest energy conformation of 1,5-hexadiene and is the one that was used in subsequent calculations.
(Your gauche conformation is less stable than both of your anti strcutures, would that hold for all gauche and anti conformations? João (talk) 12:07, 24 December 2014 (UTC))
The Ci structure was subsequently reoptimized in the B3LYP/6-31G* level of theory to give the following values:
Energy = -234.626827 a.u.
Point group: Ci
Based on this calculation the reactant was found to be higher in energy than in the previous calculation (I see a smaller (more negative) value João (talk) 12:07, 24 December 2014 (UTC)). This is due to the fact that the Density Functional Theory, of which B3LYP is an application, accounts for electron correlation, while the Hartree-Fock method that was applied first doesn't. Slight differences in bond lengths and angles are observed but the point group of the molecule remained the same.
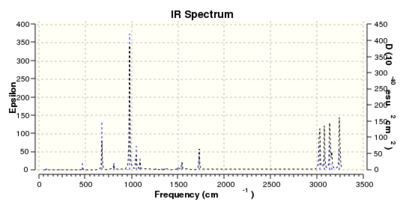
A frequency calculation was subsequently run on the optimized molecule (B3LYP/6-31G*)
(Were all frequencies non-imaginary? Is that relevant? João (talk) 12:07, 24 December 2014 (UTC)). Based on this calculation an IR spectrum prediction for the molecule was obtained. Also, the following values of energy were determined:
Sum of electronic and zero-point Energies= -234.416244 a.u. (0 K)
Sum of electronic and thermal Energies= -234.408954 a.u. (298.15 K)
Sum of electronic and thermal Enthalpies= -234.408010 a.u. (298.15 K, 1 atm)
Sum of electronic and thermal Free Energies= -234.447850 a.u. (298.15 K, 1 atm)
Optimising the transition state structures
Chair transition state
In order to determine the chair transition state structure, an allyl fragment was drawn and optimised. The fragment was then used to build an approximate structure for the transition state as shown on the right.
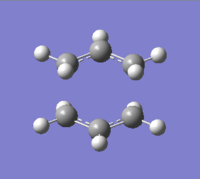
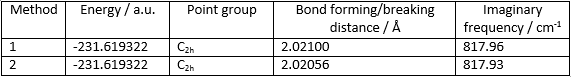
The distance between the two fragments was originally adjusted to 2.20000 Å. The structure was then optimised with two different methods.
In method 1 the structure was optimised to a transition state (Berny) and a frequency calculation was performed. The calculation showed one imaginary frequency of magnitude 817.93 cm-1. The animation of this vibration corresponds to the Cope rearrangement reaction taking place.
In method 2, the bond forming/breaking distances were frozen at 2.20000 Å and the structure was optimised to a minimum value of energy. Then the bond forming/breaking distances were optimised as well by differentiation. From the two methods very similar results were obtained.
Boat transition state
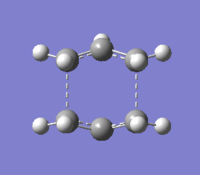
The boat transition state structure was determined by running a QST2 calculation. The reactant and the product were specified and the transition state was found by interpolation of the two. Both reactant and product were assumed to adopt the Ci conformation specified above, but some of the bond angles and dihedral angles of the molecules had to be adjusted so that the molecules could adopt a structure closer to that of the boat transition state. It was confirmed that the structure obtained corresponded to the transition state due to the fact that it had only one imaginary frequency which when animated corresponded to the rearrangement reaction taking place.
Energy: -231.602802 a.u.
Imaginary frequency: 839.75 cm-1
Bond forming distance: 2.14010 Å
Point group: C2v
By comparing the two transition structures it is apparent that the chair conformation is lower in energy and thus more stable.
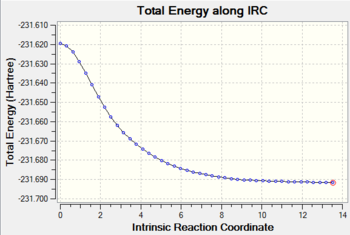
An Intrinsic Reaction Coordinate calculation was run on the chair transition state to determine which conformation the product obtained from this transition state would adopt. The reaction coordinate was computed only in the forward direction because it is symmetrical.
A total of 44 steps were required for the product to be obtained. The result of the calculation is shown below:
The product molecule is shown to adopt a C2 geometry. The middle four carbons adopt a gauche linkage. The energy of this conformation was -231.691579 a.u.
Calculating activation energy
Finally, the transition states obtained by the HF/3-21G theory were reoptimised using the B3LYP/6-31G* level of theory and a frequency calculation was also obtained. Comparing the reoptimised structures to the ones obtained before it became evident that, though the symmetry was roughly the same, the values of energy show a large difference. (Did you also check that one imaginary frequency was preserved? João (talk) 12:07, 24 December 2014 (UTC))
Based on the results it was possible to calculate the activation energy corresponding to each transition state and compare it to experimental values found in literature[1].
The activation energies were determined to be:
For the chair transition state: 34.426 kcal/mol (experimental value: 33.5 ± 0.5 kcal/mol)
For the boat transition state: 42.779 kcal/mol (experimental value: 44.7 ± 2.0 kcal/mol)
The activation energy for the boat transition state is within the error margin. For the chair transition state though the activation energy is slightly higher than the experimental prediction.
The following thermochemistry data were also obtained:
For the chair transition state:
Sum of electronic and zero-point Energies= -234.430433
Sum of electronic and thermal Energies= -234.424471
Sum of electronic and thermal Enthalpies= -234.423527
Sum of electronic and thermal Free Energies= -234.458697
For the boat transition state:
Sum of electronic and zero-point Energies= -234.418364
Sum of electronic and thermal Energies= -234.411993
Sum of electronic and thermal Enthalpies= -234.411049
Sum of electronic and thermal Free Energies= -234.447148
References
- ↑ 1.0 1.1 O. Wiest , K. A. Black and K. N. Houk, "Density Functional Theory Isotope Effects and Activation Energies for the Cope and Claisen Rearrangements", J. Am. Chem. Soc., 1994, 116, 10336–10337.DOI:10.1021/ja00101a078
- ↑ B. W. Gung , T. F. Koetzle , Z. Zhu and R. A. Fouch, "Conformational Study of 1,5-Hexadiene and 1,5-Diene-3,4-diols ", J. Am. Chem. Soc., 1995, 117, 1783–1788.DOI:10.1021/ja00111a016