Rep:Mod:phosphazenechuntminiproject
Shahania Begum
Inorganic Mini Project
Phosphonitrilic halide structures
For this mini project phosphazenes; nitrogen and phosphorous double bond containing compounds have been explored, in particular the planar trimeric structure of phosphonitrilic halides have been investigated. Where the effect of changing the halide group on the geometry, and vibrational frequencies of the compounds have been compared and evaluated. Binding effects specifically the reaction for the formation of phosphonitrilic chloride and its electronic structure has been analysed.
The four inorganic heterocycles which have been investigated are phosphonitrilic halides of formula (PNX2)3 where X= F, Cl, Br or I.

The first molecule which has been looked into is trimeric phosphonitrilic dichloride (PNCl2)3. It is synthesised by heating PCl5 and NH4Cl in chlorobenzene at around 130°C. A mixture of trimeric compounds are formed, but the trimer is relatively easy to isolate;
nPCl5 + nNH4Cl --> (NPCl2)3-10 + 4nHCl
The molecule was drawn into chembio3D where it was saved as a .gjf file then run in Gaussview. Lower level calculations were initially carried out in order to save time and processing after which higher level calculations from the "pre-optimised" molecules were computed. A DFT, calculation using B3LYP method, with a low level basis set LANL2MB with convergence criteria “opt=loose” was used to get the initial geometry right. This was followed by optimising the previously optimised structure using a better basis set and pseudo –potential with the B3LYP method, LANL2DZ with (increased electronic convergence by specifying int=ultrafine scf=conver=9 in additional keywords section. Finally the vibrational frequencies of the molecule were calculated and the full MO analysis was computed by specifying (pop=full); Below are the calculation summaries for each molecule;
| Phosphonitrilic Fluoride | Phosphonitrilic Chloride | Phosphonitrilic Bromide | Phosphonitrilic Iodide | ||||||||||||
|
|
|
| ||||||||||||
 |
 |
 |
 |
It can be seen from the jmol of the phosphonitrilic iodide that despite taking three attempts to optimise the molecules geometry starting from scatch, followed by a second optimisation the sructure failed to aromatise the planar ring. However the vibrational spectrum and electron density and molecular orbitals can still be compare to the other three structures. It is also very interesting to note that phosphonitrilic halides have not been prepared. DOI:10.1021/cr60217a004
Energies
The energies of the different compounds have been compared to see which structure is the most thermodynamically stable;
| Compound | Energy a.u | Energy kJ/mol |
| (PNF2)3 | -782.70311524 | -2054987.03 |
| (PNCl2)3 | -273.24141783 | -717395.35 |
| (PNBr2)3 | -262.56595787 | -689366.92 |
| (PNI2)3 | -251.90257441 | -661370.21 |
The stabilisation energy for the fluoride compound has a stabilisation energy almost three times as large as that of the chlorine containing compound.∆E=E(Fhigh)-E(Cllow)= 1337591.68 kJ/mol, this energy difference is huge, however it follows trend as strong unreactive P-F bonds add to the stability of the aromatic system. Whereas the change in energy differemce between the chloride and bromide energies is similar to that of the difference in energies between the bromide and iodide complexes ∆E respectively are 28028.42 and 27996.71kJ/mol.
The halogens seem to follow the usual trend of Fluoride forming the most stable of compounds, with thermodynamic stability gradually decreasing down the group.
The energy of phosphonitrilic iodide is quite close to that of phosphonitrilic bromide despite not having favourable stablisation from the aromatic ring. Perhaps the iodide complex could in fact be even closer to the energy of the bromide complex.
Charges
The NBO charges on the specific atoms of each molecule have been looked at to see if any insight could be gained as to the localisation of electron density on the molecules and what effect the electron withrawing halogens have on each structure;
| Phosphonitrilic Fluoride | Phosphonitrilic Chloride | Phosphonitrilic Bromide | Phosphonitrilic Iodide |
 |
 |
 |
 |
On the fluoride structure the natural positive charge +2.427 appears to be at the phosphorous atoms with a lot of the electron density, three times the amount in the aromatic ring on each nitrogen atom -1.642 compared to the negative charge on the electron with drawing fluorine atoms -0.510.
In comparison, on the chloride containing compound the, the chlorine atoms are less electron with drawing than fluorine with a charge of -0.170 which does not draw electron density away from the ring and phosphorous as strongly as fluorine. Phosphorous atoms are now less electropositive with a charge of +1.642. The electron density appears more evenly spread across the ring, as less charge is being drawn away from the ring.
In the phosphonitrilic bromide, the negative charge on the Br atoms is even less at -0.071 and again this decreases ever closer to having no charge for the iodine atoms. Overall as the electron withdrawing nature of the halogens down the group decreases there is a better charge distribution and balance between P and N atoms in the ring. Due to the Iodine calculations not fully optimising the geometry of the phosphonitrilic iodide the charges for each group of atoms are not the same however the symmetry of the molecules suggests that each atom of the same element should have the same charge. The bond strength is greater as the element is more electronegative such is the case for fluoride and hence this is the most stable structure by far as the comparisson of energy comfirmed.
Geometries
The Iodide molecule took the longest time to compute; the larger the halide atoms the longer it took for the calculations to run and for the structures to be optimised.
| Bond lengths (Å) | Phosphonitrilic fluoride | Literature | Phosphonitrilic chloride | Literature |
| P-X | 1.67 | 1.51 | 2.21 | 1.986 |
| P-N | 1.67 | 1.56 | 1.68 | 1.577 |
| Bond angles (°) | Phosphonitrilic fluoride | Literature | Phosphonitrilic chloride | Literature |
| X-P-X | 90.0 | 99.0 | 100.8 | 101.9 |
| N-P-N | 117.9 | 119.0 | 117.0 | 118.5 |
| P-N-P | 122.1 | 121.0 | 123.0 | 121.1 |
For the Chloride compound the computed P-Cl is 0.22Å larger than that of literature. Otherwise the calculated bond lengths and angles are close to those of literature. The general trend appears to be going down group 17 as the halogen atom becomes heavier and larger in size the length of the P-X bond becomes longer and the X-P-X angle widens due to sterics and possible electron-electron repulsion.
| Bond lengths (Å) | Phosphonitrilic Bromide | Literature | Phosphonitrilic Iodide |
| P-X | 2.41 | 2.20 | 2.63 |
| P-N | 1.69 | 1.60 | 1.72 |
| Bond angles (°) | Phosphonitrilic Bromide | Literature | Phosphonitrilic Iodide |
| X-P-X | 102.1 | 102.5 | 103.9 |
| N-P-N | 116.2 | 118.1 | 116.6 |
| P-N-P | 123.8 | 124.3 | 120.4 |
No literature could be found for the Iodide trimer.It is interesting to note that the average P-N bond length is ~1.73Å and the P=N bond length is shorter and observed between 1.58-1.61Å. On average in the phosphonitrilic conjugated trimers the P-N bond is predicted to be 1.68Å. This suggests the P-N bond is inbetween a single and a double bond and is part of an aromatic system similar to that of benzene.P-N bond length increases through F to I which should cause a loss of aromaticity in the ring. This may be the reason why the Iodide molecule did not conform to a planar ring system after optimising and is heading towards a chair conformation.
IR specta, Vibrational analysis
Below are possible ring stretching vibrations that can give rise to absorption frequencies in the IR spectrum;

Below is the IR spectrum of phosphonitrilic fluoride and the assignment of the vibrational freqeuncies alongside literature values. DOI:http://hdl.handle.net/10042/to-1964

| Assignments | Vibrational Frequencies (cm-1) | Literature |
| E'PF2wag or deformation, degenerate | 382s | 385s |
| A1'trigonal ring deformation | 614m | 621m |
| E' sym. PF2 stretching degenerate | 828vs | 795vs |
| A'sym. PF2 stretching in phase | 811w | 862w |
| E'ring stretching, degenerate (II) | 1128vs | 1113vs |
It can be seen that the stretching vibrations for the symmetrical PF2 lies below 885cm-1. The P-F stretch absorbs more strongly at a higher frequency compared to the other P-Cl, P-Br and P-I stretches which absorb less strongly at lower frequencies as can be seen below.
The IR spectrum of phosphonitrilic chloride and the vibrational frequency assignments. DOI:http://hdl.handle.net/10042/to-1965

| Assignments | Vibrational Frequencies (cm-1) | Literature |
| E'ring elongation | 392s | 336s |
| E' sym. PCl2 stretching, degenerate | 507vs | 527vs |
| A2" antisym. PCl2 stretch in phase | 611vs | 612vs |
| A1'sym. PCl2 trigonal ring deformation | 695w | 695w |
| E"antisym. PCl2 stretch, degenerate | 930w | 915w |
The PCl2 stretching frequency is much lower at 614cm-1 compared to the fluorine P-F stretch. The ring stretching vibrationas are also reported in literature which have not been computed within this range of frequencies as they are theoretically inactive;
An E' ring stretch, deenerate (II)reported at 1218vs An E' ring stretch, deenerate (III)reported at 1218m An A2' trigonal stretching (IV) at 1368m
IR spectrum of phosphonitrilic bromide DOI:http://hdl.handle.net/10042/to-1963

IR spectrum of phosphonitrilic iodide DOI:http://hdl.handle.net/10042/to-1958

The above spectra are similar to that of the phosphonitrilic chloride apart from the fact that the hypothetical iodide molecules predicted vibrational frequencies are a little different. There are two frequencies around 1115 and 1168cm-1 from E' ring stretching, which are no longer degenerate as the ring diverges from planarity. The predicted frequencies are quite close to literature.
Phosphonitrilic chloride (PNCl2)3MO analysis:
DOI
The planar ring system showed aromaticity, a delocalised pi conjugated system similar to that of benzene. The cyclic trimer has a planar six-membered ring structure. All of the P-N bond lengths are identical and are shorter than normal P-N single bonds. This suggests multiple delocalised bonding.
The observed structure is best described in terms of (P)dπ-(N)pπ overlap. Assuming sp2 hybridisation at N and sp3 hybridised P, a σ-bonded framework may be built up which makes use of two nitrogen sp2 and four phosphourous sp3 orbitals. A further ‘non-bonding’ sp2 orbital is occupied by a lone pair, while the pz orbital is available for further π-interaction dxy and dyz orbitals of phosphorous.
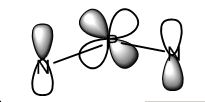
The Mo’s for this molecule were examined and found to be very interesting, The Mo’s have been looked at to try and explain this aromatic, stable and unreactive nature of this species.
| Lumo+3 | Lumo+2 | Lumo+1 |
 |
 |
 |
| Lumo | Homo | Homo-1 |
 |
 |
 |
| Homo-2 | Homo-3 | Homo-4 |
 |
 |
 |
The molecular orbitals displaying the most delocalised electron density across the entire ring thereby supporting the evidence that the phosphornitrilic ring is aromatic in nature.
| MO 4 | MO 9 | MO 17 |
 |
 |
 |
Conclusion
The results demonstrate that despite the structures being the same for the phosphonitrilic halides, by changing the halides the geometry of the molecule changes greatly, affecting the planarity and aromaticity of the conjugated ring system with the bromide compound and theoretical iodide halides ring converging to a chair conformation. The nitrogen and phosphorous double bond containing compounds have been explored,It was very interesting to see the difference in bond lengths and angles Vibrational effect. The molecular orbitals were also explored to see where the aromaticity arises from in the chloride compound and what influecne the NBO charges has on this.
References
Fluoride Geometry literature:Jim Durig, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. Volume 61, Issue 7, May 2005, Pages 1499-1503 DOI:10.1016/j.saa.2004.11.058
Chloride Geometry literature:Acta Crystallographica Section B, Acta Cryst. (2006). B62, 321–329 DOI:10.1107/S0108768106000851
Bromide Geometry Literature:EDOARDO GIGLIO AND RAFFAELLA PULITI, Acta Cryst. (1967). 22, 304
Fluoride and Chloride phosphonitrile trimer vibrational frequencies references:Phosphonitrilic Derivatives. Part VIII: The Vibrational Spectra of Phosphonitrilic Halides. By A. C. CHAPMAN and N. L. PADDOCK.J. Chem. Soc., 1962, 635 - 645, DOI: 10.1039/JR9620000635
