Rep:Mod:phj1145
Introduction
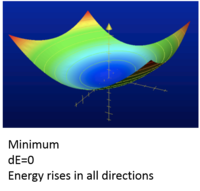
Potential Energy Surface is showing a plot of single point calculation of each possible structure of a molecule. A minimum point on Potential Energy Surface is when dE=o and where energy rises all directions as shown from figure 1.
Nf710 (talk) 00:00, 18 November 2016 (UTC) A single point energy is just that a point not a surface. nice diagram though
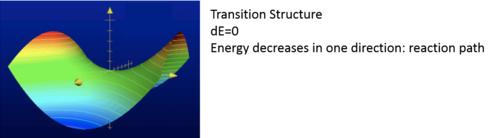
In this experiment all the structures are optimised to minimum because a transition state is also when dE=0. Transition state is when dE=0 , where energy decreases in one direction as shown from figure 2.
The frequency of the minimised point is calculated if it results in one imaginary frequency (k is negative) the point is transition state. This imaginary frequency shows vibration that corresponds to the reaction coordinate. If all the vibrational frequencies are real, this confirms the structure is a minimum. And if a frequency calculation has one imaginary frequency, this confirms the structure is a transition state.[1]
Nf710 (talk) 00:06, 18 November 2016 (UTC) NIce use of diagrams here. your notation was abit off. it should be DE/d(degree of freedom)
Reaction of Butadiene with Ethylene
MO diagram for the formation of the butadiene and ethene transition state

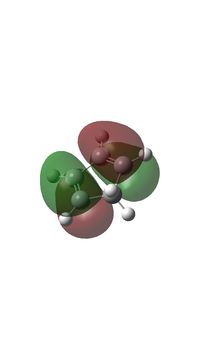
- The HOMO of butadiene which has g symmetry overlap with LUMO of ethylene which is also g symmetry. And therefore the orbital interaction is g-g and the orbital overlap integral is non-zero and the reaction is allowed.
- The LUMO of butadiene which has u symmetry overlap with HOMO of ethylene which is also u symmetry. And therefore the orbital interaction is u-u and the orbital overlap integral is non-zero and the reaction is allowed.
- For the case g-u interaction the orbital overlap integral is zero.
- The g-g interaction (HOMO of butadiene and LUMO of ethylene) causes one bonding (MO 15) and one antibonding orbital (MO 17) and the antibonding orbital is HOMO of transition state (and also product).
- The u-u interaction (LUMO of butadiene and HOMO of ethylene) causes one bonding (MO 16) and one antibonding orbital (MO 18) and the antibonding orbital is LUMO of transition state (and also product).
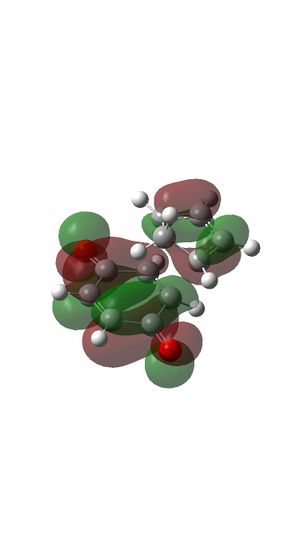
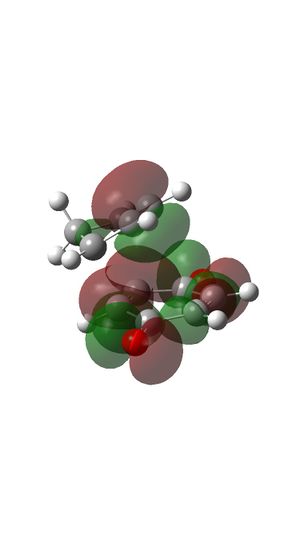
HOMO and LUMO of reactants
|
| ||||||
|
LUMO OF Ethylene
|
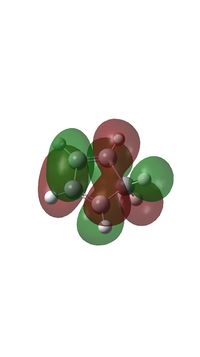
- HOMO of butadiene has the same symmetry with LUMO of ethylene and they form MO 17 (HOMO) and MO 15 of transition state.
- LUMO of butadiene has the same symmetry with HOMO of ethylene and they form MO 18 (LUMO) and MO 16 of transition state.
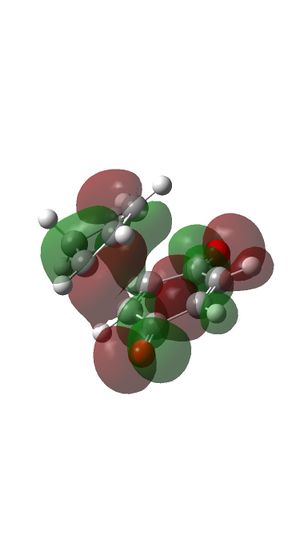
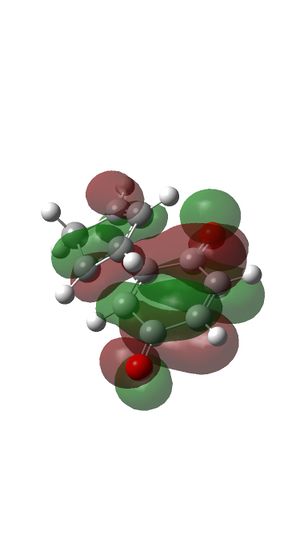
MOs of Transition state
|
| ||||||
|
|
- MO 15 is an occupied bonding orbital formed with HOMO of butadiene and LUMO of Ethylene.
- MO 16 is an occupied bonding orbital formed with LUMO of butadiene and HOMO of Ethylene.
- MO 17 is an occupied antibonding orbital formed with HOMO of butadiene and LUMO of Ethylene (HOMO).
- MO 18 is an unoccupied antibonding orbital formed with LUMO of butadiene and HOMO of Ethylene (LUMO).
Measurements of the Bond lengths
- Bond lengths of the Reactants

- Bond lengths of the Transition state
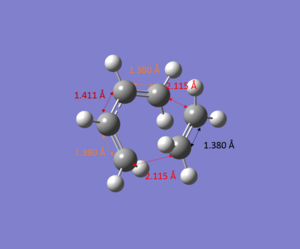
- Bond lengths of the Product

- The Van der Waals Radius of the C atom is 1.70 Å 2 and the distance for two bonds forming for the reactants are 3.14 Å which are longer than 2*van der Waal radius (2.40 Å). For the distance of two bonds forming is less than van der Waals diameter as 2.115 Å. For the product the distance of these two bonds are decreased to 1.54 Å which is typical sp3 c-c bond length.3
- The bond lengths of c-c double bonds are increased as the reaction proceeds. The c-c double bonds for butadiene are 1.335 Å, for transition state they are increased to 1.38 Å and for the product they become a single bond with bond length of 1.50 Å. The c-c double bond for ethylene is 1.327 Å and it increased to 1.380 Å at the transition state and it becomes single bond for the product with bond length 1.546 Å.
- The c-c single bond length of butadiene decreased as the reaction proceeds. The initial bond length is 1.468 Å, and it decreased to 1.411 Å at transition state and for the product its bond length is 1.338 Å and it becomes a double bond.
Vibration
Vibration of transition state (Negative Frequency)
Figure 15: Vibration corresponds to the reaction path at the transition state |
- This imaginary frequency shows vibration that corresponds to the reaction coordinate.
Vibration the lowest positive frequency
Figure 16: Vibration of the lowest positive frequency |
- The vibration of the negative frequency is showing that formation of two bonds is synchronous.
- The vibration of the lowest positive frequency is asynchronous.
Nf710 (talk) 00:10, 18 November 2016 (UTC) This section was dont very well and you showed a good understanding of everything. Nice use of jmols
Reaction of Benzoquinone with Cyclopentadiene
Energies
| TS structure | Reaction barriers (in KJ/mol) | Reaction energies (in KJ/mol) |
|---|---|---|
| Endo | 125.40 | 11.98 |
| Exo | 132.09 | 12.93 |
Table 1: Reaction barriers and Reaction energy of Endo and Exo products.
- The reaction barrier of the exo-transition state is higher showing that the endo-product is kinetically favourable products.
- The endo-product is lower in energy as shown in table 1, and therefore endo-product is also thermodynamically favourable product.
- Overall endo-product is favourable. Diels Alder reaction is often endo-stereo selective and the reaction is kinetically controlled.
Nf710 (talk) 00:19, 18 November 2016 (UTC) You have come to correct conclusion well done. However you have used the reactant energies from infinite separation. when you should ahve used the reactants on the IRC and then optimized it down. as there is a separation interaction energy. you would then have 2 different reaction energies for the endo and exo. But you still came to the correct conclusion.
MO diagram for the Diels-Alder reaction
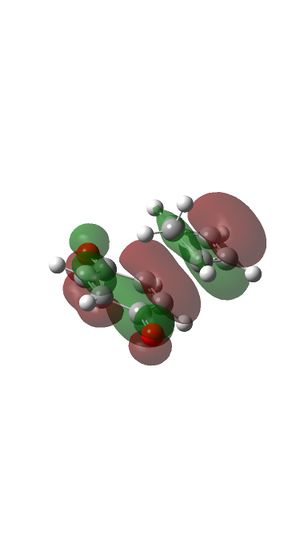
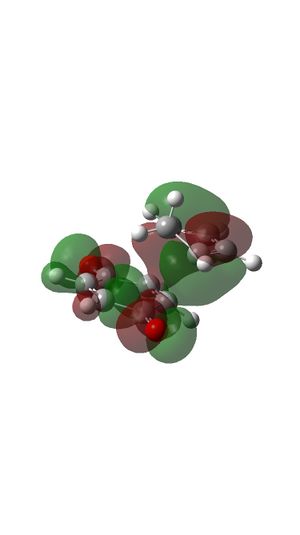
- The reaction is normal demand Diels Alder reaction. The two double bonds of cyclopentadiene are relatively electron rich and the double bond of benzoquinone is relatively electron poor because of the electron withdrawing carbonyl groups are attached. And because these electron withdrawing groups on dienophile, the energy is much lower compare to ethylene which is shown at the MO diagram of Endo- and Exo- transition state. (Figure 21 and 26)
- Endo product is favourable because endo-transition state has a secondary orbital interaction as well as primary orbital interaction where exo-transition state only has primary orbital interaction. The presence of secondary orbital interaction lowers the reaction barrier and makes the reaction towards the endo-product faster.4
- Relevant MO of reactants
- MO diagram for EXO-transition state.

- MO diagram for ENDO-transition state.

Nf710 (talk) 00:21, 18 November 2016 (UTC) Well done , your MOs are excellent and you showed the secondary orbital overlapp excellently with these. One improvement would have been to use jmols So I could properly inspect the secondary orbital overlap.
Diels-Alder vs Cheletropic
Reaction Energies and Reaction Barriers
| Reaction types | Reaction Barriers (in KJ/mol) | Reaction Energies (in KJ/mol) |
|---|---|---|
| DA, Endo | 82.80 | -142.61 |
| DA, Exo | 86.78 | -98.63 |
| Cheletropic | 105.13 | 11.31 |
(I don't know why you have a positive reaction energy for the cheletropic reaction. It can be useful to include log files or JMols so we can see what happened Tam10 (talk) 15:06, 9 November 2016 (UTC))
Table 2: Reaction barriers and reaction energies of Diels Alder reaction and cheletropic reaction
Reaction profiles

- Endo-transition state is lower and therefore endo product is kinetically favourable and also as its product is lower in energy endo-product is both kinetically and thermodynamically favourable product.
- Cheletropic has the highest reaction barriers and higher reaction energies. And also the energy of product is higher than that of reactants.And therefore it is unfavourable reaction.
Intrinsic Reaction Coordinate
IRC of Endo

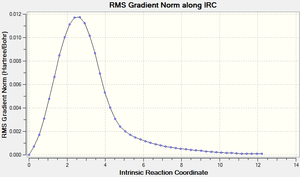
- The reaction barrier of Endo is the lowest out of Exo and Cheletropic as shown in the calculation and two graphs from IRC figure 32 and 33. During the reactions, the 6-membered ring becomes aromatic and this lowers the energy of product.
IRC of Exo
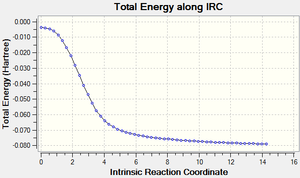
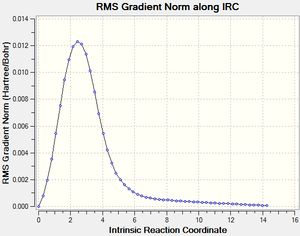
IRC for Cheletropic Reaction
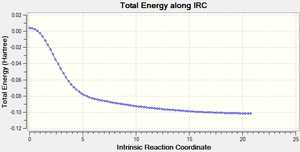
(No mention of the instability of xylylene Tam10 (talk) 15:06, 9 November 2016 (UTC))
Reference
1. Calculating Molecular Geometries, Quantum Mechanics 3, Michael Bearpark
2. http://periodictable.com/Properties/A/VanDerWaalsRadius.v.log.html (accessed on 27th Oct 2016)
3. https://en.wikipedia.org/wiki/Bond_length (accessed on 1st November 2016)
4. https://en.wikipedia.org/wiki/Frontier_molecular_orbital_theory#Cycloadditions (accessed on 2nd November 2016)