Rep:Mod:pg1910za
Diels Alder Cyclo-addition
Here were will attempt to model the TS and the reactions energies of a massively important reaction in the world of chemistry.
Starting Simple
Here we are going to take on molecule of ethene and one of cis-butydiene, and using a semi-emperical method with the AM! basis set, we shall model the molecular orbitals of the two in the hopes of better understanding the HOMO/LUMO orbitals and how they might interact.
First using the same method and basis set, we must optimise the molecule.
| File Name | PG_DiENE_HE_AM1_OP |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RAM1 |
| Basis Set | ZDO |
| Charge | 0 |
| Spin | Singlet |
| E(RAM1) | 0.04879717 a.u. |
| RMS Gradient Norm | 0.00000068 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.0415 Debye |
| Point Group | C2V |
| Job cpu time: | 0 days 0 hours 0 minutes 18.0 seconds. |
| File: | File:PG DiENE HE AM1 OP.log |
Item Value Threshold Converged?
Maximum Force 0.000001 0.000015 YES
RMS Force 0.000000 0.000010 YES
Maximum Displacement 0.000010 0.000060 YES
RMS Displacement 0.000004 0.000040 YES
Predicted change in Energy=-1.294434D-11
Optimization completed.
-- Stationary point found.
| |
Energy Calculation.
File:PG DiENE HE AM1 EN2.log
| Orbital | Ethene | Butadiene |
|---|---|---|
| HOMO | 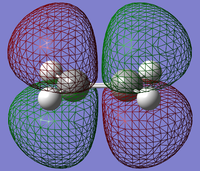 |

|
| LUMO |  |

|
From the diagrams We should expect the LUMO of the Diene to interact with the HOMO of the Ethene (both of which are symmetric in respect to the plane perpendicular to their central bond) , and the HOMO with the LUMO therefor both can be allowed transitions (antisymmetric with respect to plane).
Transition state, Method 1
A guess was made of the Transition state by taking the gaussian template structure of bicyclo-2,2,2-octane, removing one of the -CH2-CH2- units,


| File Name | PG_RXN_T3_AM1_OP_TSb4 |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RAM1 |
| Basis Set | ZDO |
| Charge | 0 |
| Spin | Singlet |
| E(RAM1) | 0.11165465 |
| RMS Gradient Norm | 0.00000111 |
| Imaginary Freq | 1 |
| Dipole Moment | 0.5605 |
| Point Group | C1 |
| Job cpu time: | 0 days 0 hours 0 minutes 54.1 seconds. |
| File: | File:PG RXN T3 AM1 OP TSb4.log |
Item Value Threshold Converged?
Maximum Force 0.000003 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000144 0.001800 YES
RMS Displacement 0.000038 0.001200 YES
Predicted change in Energy=-4.160235D-10
Optimization completed.
-- Stationary point found.
| |
In the Orbital Diagram of the transition state we see that the interaction seems to be for the LUMO of Ethene and the HOMO of the Diene, this makes sense when remembering that stability increases as conjugation increases, therefor the HOMO of Diene will have a lower energy the the HOMO of Ethene.
Calculating with a higher basis set
Issues arose when trying to run the above using DFT/6-31Gd, as what would often happen is that either only a single bond would form between the two molecules, or the Ethene would completely reorientate to form some bizzar interaction with just one of the double bonds of the Diene-
The Decision was made to run the calculation using QTS2 as it allows us to define the product, therefore forcing the two desired bonds to be formed, in a concerted manner in the transition state.
| File Name | PG_RXN_T2_OP_qts_log_87530 |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -234.54389656 a.u. |
| RMS Gradient Norm | 0.00000068 a.u. |
| Imaginary Freq | 1 |
| Dipole Moment | 0.3943 Debye |
| Point Group | C1 |
| Job cpu time: | 0 days 0 hours 4 minutes 55.2 seconds. |
| File: | File:PG RXN T2 OP qts.log |
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000032 0.001800 YES
RMS Displacement 0.000008 0.001200 YES
Predicted change in Energy=-3.907341D-11
Optimization completed.
-- Stationary point found.
| |
| Energy File: | File:PG RXN T2 OP qts EN.log File:PG RXN T2 OP qts EN.fchk |
| Frequency at 0K | File:PG RXN T2 OP qts FR0k.log |
| Frequency at 298K | File:PG RXN T2 OP qts FR298k.log |
| Parameter | Distance |
|---|---|
| Van der waals radius of Carbon |
1.7 Å |
| C--C Bond length | 1.54 Å |
| C==C Bond Length | 1.34 Å |
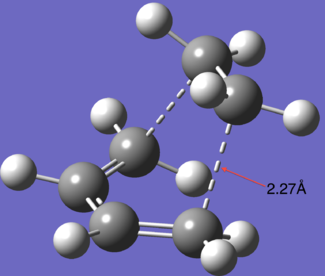
| TS C C distance | 2.27 Å |
What we can see with the above information is that the partially formed bond is much to long to be considered a formal bond (comparing it to the C--C and C==C bond lengths) but the fact that the distance is less then twice the van der waals radius of carbon we can conclude that there is some kind of interaction taking place between the fragments.
 |
 |
Here we see that for the Imaginary frequency, that corresponds to the vibration associated to the formation of the bonds in the reaction, both of the bonds are being formed at the same time. This agrees with the accepted idea that the reaction is a pericyclic process.
The lowest positive frequency is just a vibration of the structure and not in itself associated with bond forming, if anything the motion would act to disrupt the bond formation as the twisting motion would break the symmetry of the process.
Cyclohexa-1,3-diene reacting with maleic anhydride
 Seen above is the reaction between Cyclohexadiene and maleic anhydride.
By using computation methods we hope to be able to compare the relitive stabilities between the endo and exo conformers of the reaction product.
Seen above is the reaction between Cyclohexadiene and maleic anhydride.
By using computation methods we hope to be able to compare the relitive stabilities between the endo and exo conformers of the reaction product.
All the calculations have first been run at HF/3-21G and then reoptimised at DTF/6-31G* all properties disscussed, unless otherwise stated, will be from the final optimisation (6-31G*)
Transition states
Due to issues encountered in earlier sections, It was decided to use the QTS2 method to calculate the TS.
| Exo | Endo | |
|---|---|---|
 |

| |
| File Name | PG_ExoRxn_FROP_DFT631GdT3_log_87649 | PG_EndoRxn_FROP_DFT631Gd_log_87564 |
| File Type | .log | .log |
| Calculation Type | FREQ | FREQ |
| Calculation Method | RB3LYP | RB3LYP |
| Basis Set | 6-31G(d) | 6-31G(d) |
| Charge | 0 | 0 |
| Spin | Singlet | Singlet |
| E(RB3LYP) | -612.67931096 a.u. | -612.68339677 a.u. |
| RMS Gradient Norm | 0.00000166 a.u. | 0.00000435 a.u. |
| Imaginary Freq | 1 | 1 |
| Dipole Moment | 5.5502 Debye | 6.1143 Debye |
| Point Group | C1 | C1 |
| Job cpu time: | 0 hours 25 minutes 37.5 seconds. | 0 hours 26 minutes 2.5 seconds. |
| File: | File:PG ExoRxn FROP 631GdT3.log | File:PG EndoRxn FROP 631Gd.log |
| Reactant | ||
| Exo | Endo | |
|---|---|---|
| TS | 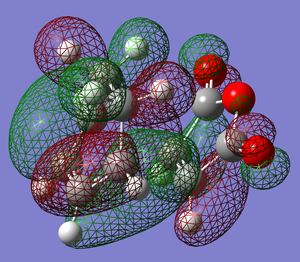 |
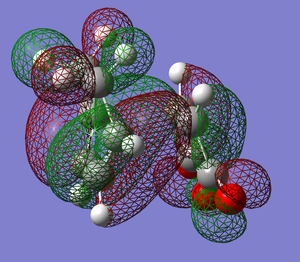
|
| Prod |  |
|
Possible strain seen in the endo product
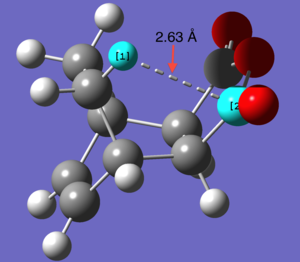
| perameter | Length ( Å) |
|---|---|
| Van der waals radius of Hydrogen |
1.2 |
| Van der waals radius of Carbon |
1.7 |
| Distance between H C | 2.63 |
What we see when looking at the distance between the Hydrogen and Carbon (indicated in the picture to the right) is that distance between the two atoms is less than the sum of their van der waals radii. While the degree to which they interact would be hard to determine as, especially for a carbonyl carbon, there will be effects on the van der waals radius by neighboring atoms.
| B3LYP/6-31G* | |||
|---|---|---|---|
| Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | |
| at 0 K | at 298.15 K | ||
| Maleic anhydride | -379.28954470 | -379.233657 | -231.445306 |
| Cyclohexadiene | -233.41893632 | -233.296105 | -379.228473 |
| ExoTS | -612.67931096 | -612.498013 | -612.487662 |
| EndoTS | -612.68339677 | -612.502141 | -612.491787 |
| EndoProd | -612.75578550 | -231.539540 | -231.532566 |
| EndoProd | -612.75829021 | -231.539540 | -231.532566 |


