Rep:Mod:pdg08mod3
Module 3: Praveen Gnanasambanthan 00557484
Cycloaddition transition states
The Cope Rearrangement of 1,5 Hexadiene
Introduction

The 3,3 sigmatropic Cope rearrangement reaction of 1,5 hexadiene was studied by computational methods. The objective of this experiment was to determine whether this rearrangement occurred via the boat or the the chair configuration transition states. Conformers were analysed and then sent to scan. The most stable structures were further optimised and then were put through frequency analysis.
Optimisation
There are various conformations of 1, 5-hexadiene due to free bond rotation between carbon atoms 2,3,4,5. Since this would produce abundant conformations, the conformers which were eclipsed such as synperiplanar were not modelled since they are expected to have high energies than those of the gauche and antiperiplanar configurations. These configurations were cleaned up so their structure could be optimisied using the Hatree-Fock Method with a 3-21G. From this it was observed that the “Anti 1”, “Anti 2” and the “Gauche 3” structures were the most stable conformers as can be seen from the data below.
| Conformer
& Jmol |
Optimised
Hartree Energy |
Relative
Energy [kcalmol-1] |
Point
Group |
|---|---|---|---|
| [1] | -231.69260 | 0.04 | C2 |
| [2] | -231.69254 | 0.08 | Ci |
| [3] | -231.68907 | 2.25 | C2h |
| [4] | -231.69097 | 1.06 | C1 |
| [5] | -231.68772 | 3.10 | C2 |
| [6] | -231.69167 | 0.62 | C2 |
| [7] | -231.6922 | 0.00 | C1 |
| [8] | -231.69153 | 0.71 | C2 |
| [9] | -231.68962 | 1.91 | C1 |
| [10] | -231.68916 | 2.20 | C1 |
|}
No immediate trend is noticed through the optimisation. It is not clear whether the gauche or the antiperiplanar configuration is more stable. It is slightly surprising, at first glance that the 3rd gauche confirmation is the most stable since for most other organic molecules the antiperiplanar as is the case for butane [11]. However the favourable interactions of the two π orbitals where the two double bonds are near each other due to the gauche configurations. This lowers the energy more, relative to the steric stabilization that the antiperiplanar structure offers. [12]
The three most stable structures were further optimised by using a DFT method with a 31-G* basis set, to give a more accurate and better optimised molecule due to electron denisity and correlation being taken into account.| Conformer & Jmol | Optimised
Hartree Energy |
Relative
Energy [kcalmol-1] |
Point
Group |
|---|---|---|---|
| [13] | -234.61178 | 0.00 | C2 |
| [14] | -234.61171 | 0.04 | Ci |
| [15] | -234.61132 | 0.29 | C1 |
This optimisation shows that the "Anti 1" conformation is actually the most stable structure, while the "Gauche 3" structure is the least stable of structures. This is put down to the better overlap of the allyl moieties which cannot be done in the gauche configuration. In addition to this the stearic hindrance caused is kept to a minimum in the anti configurations. Further lowering the energy. This optmisation however is not too different to the Hatree Fock Method
Vibrational analysis of the 1,5 hexadiene
From the previous module it was established that all second derivatives of energy with respect to the geometrical coordinates had to be positive, so the frequencies are real and the molecule is at a minimum. The "Anti 2" conformation is only done, this is due to the fact that all conformers are the same due to free bond rotation. A negative frequency would imply the molecule is not at a minimum and there is energy for the molecule to lose so by doing this analysis [16] ensures that the molecules are fully optimised to the most stable structure. The IR spectrum is shown below.
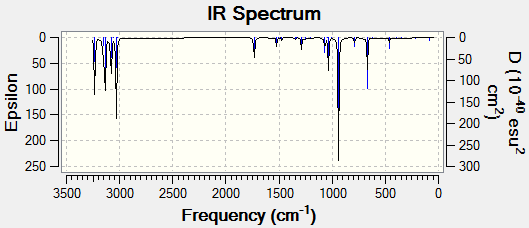
The true minimum was found due to the fact that no negative frequencies appeared in the output file. The two most significant stretches are displayed above. The other stretches were not tabled since C-C stretches and C-H stretches are common information to all chemists. The frequencies for the double bonds differ to the literature values [17] [18] while the values obtined by the calculations are between 1730cm-1 and 1740cm-1, the literature values are 1643cm-1 and 1830cm-1.
Thermochemical analysis of the 1,5 hexadiene
By analysing the vibrational analysis file, the thermochemical analysis data is obtained.
Zero-point correction= 0.142491 (Hartree/Particle)
Thermal correction to Energy= 0.149847 Thermal correction to Enthalpy= 0.150791 Thermal correction to Gibbs Free Energy= 0.110882 Sum of electronic and zero-point Energies= -234.469212 Sum of electronic and thermal Energies= -234.461856 Sum of electronic and thermal Enthalpies= -234.460912 Sum of electronic and thermal Free Energies= -234.500821
References
- ↑ http://hdl.handle.net/10042/to-13686
- ↑ http://hdl.handle.net/10042/to-13687
- ↑ http://hdl.handle.net/10042/to-13688
- ↑ http://hdl.handle.net/10042/to-13689
- ↑ http://hdl.handle.net/10042/to-13690
- ↑ http://hdl.handle.net/10042/to-13691
- ↑ http://hdl.handle.net/10042/to-13692
- ↑ http://hdl.handle.net/10042/to-13693
- ↑ http://hdl.handle.net/10042/to-13694
- ↑ http://hdl.handle.net/10042/to-13695
- ↑ H. Rzepa, 2nd Year Conformational Analysis Course, 2011, p. 'Alkanes',http://www.ch.ic.ac.uk/local/organic/conf/
- ↑ Gung, B. W.; Zhu, Z.; Fouch, R.; J. Am. Chem. Soc., 1995, 117, 1783-1788. DOI:10.1021/ja00111a016
- ↑ http://hdl.handle.net/10042/to-13696
- ↑ http://hdl.handle.net/10042/to-13697
- ↑ http://hdl.handle.net/10042/to-13698
- ↑ http://hdl.handle.net/10042/to-13807
- ↑ J. Coates, Encyclopaedia of Analytical Chemistry, R. A. Meyers (Ed.), 2000, pp. 10815-10831
- ↑ http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi