Rep:Mod:pdg08mod1
Molecular Modelling
Introduction
Molecular modelling is a very useful tool in chemistry, permitting chemists to analyse chemicals and chemical reactions before any experimental work is done. This useful tool helps with solving problems and creating hypotheses in a very efficient manner. Things such as the energy, symmetry, transition state and the way the molecule reacts is modeled. UV-Vis, IR, various NMR spectra are predicted for the molecules. These techniques and tools are used to solve and explain the various problems listed below.
Molecular Mechanics
The Hydrogenation of a cyclopentadiene Dimer
The kinetic and thermodynamic products from the dimerisation of cyclopentadiene
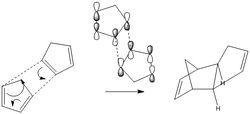
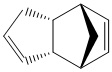
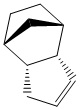
Cyclopentadiene is a very common compound used in a variety of chemical reactions. Its versatility has seen in being used and applied in many branches of chemistry. The reagent is most commonly found in organometallic chemistry where it acts as a ligand with a multi-hapticity e.g. ferrocene. In addition to this it this reagent is important in alkene polymerisation, forms the ligand part of the Zeigler-Natta catalysts. [1] Cyclopentadiene also has the cability of under going 4πS + 2πS cyclo addition reactions. The reaction below that is studied is based on one such reaction where two cyclopentadienes combine.
There are two diastereoisomers that form from this, usually undesired, reaction, the exo isomer (1) and the endo isomer (2). The endo product predominates under normal conditions however at higher temperatures the exo diastereoisomer is the major product, as the dimer switches from endo to exo. The endo product is roughly 2kcal mol-1 higher in energy than exo product. This indicates the the endo product is the kinetic product while the exo product is the thermodynamic product. The main cause of this difference as established by MM2 calculations was the bond torsion. When inspecting the molecules it is clear to see that there is a significant repulsion effect in the endo molecule caused by the by the carbons that are situated 1,4 to each other. Regardless of this torsion effect. The endo diastereomer forms at a faster rate and this can be explained by the transition state. As the two diagrams of the formation of the dimers illustrate, there is a significant stabilisation interactions in between the 4π LUMO and the 2π. These means the transition state and therefore the activation energy to be overcome for the formation of the endo cyclpentadiene dimer is of a lower energy than that of the exo dimer and thus at lower temperatures this endo product is more likely to form.
 |
 | |||
| Energies/ kcal mol-1 | ||||||||
| Stretch | Bend | Stretch-Bend | Torsion | Non 1,4 Van der Waals | 1,4 Van der Waals | Dipole-Dipole | Total | |
| Exo dimer (1) | 1.2850 | 20.5783 | -0.8382 | 7.6559 | -1.4167 | 4.2346 | 0.3775 | 31.8765 |
| Endo dimer (2) | 1.2497 | 20.8492 | -0.8354 | 9.5109 | -1.5434 | 4.3190 | 0.4475 | 33.9975 |
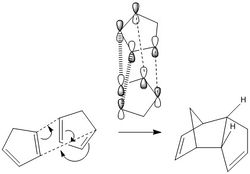
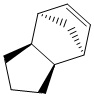
The hydrogenation of the endo diastereomer of the cyclopentadiene dimer
| Energies/ kcal mol-1 | ||||||||
| Stretch | Bend | Stretch-Bend | Torsion | Non 1,4 Van der Waals | 1,4 Van der Waals | Dipole-Dipole | Total | |
| Possible structure (3) | 1.2659 | 19.8063 | -0.8276 | 10.8698 | -1.2207 | 5.6394 | 0.1621 | 35.6953 |
| Possible structure (4) | 1.0806 | 14.5311 | -0.5468 | 12.5055 | -1.0690 | 4.5152 | 0.1406 | 31.1572 |
The hydrogenation of only one of the double bonds inevitably leads to the formation of two products. These are labeled as (3) and (4) to distinguish between the two. By inspection of the table below the more energetically stable product is structure 4. The difference in energy is attributable to the difference in the "bend" energy. This is the degree at which the bonds twist or move out of shape to accommodate for the shape of the molecule, the angular strain.
The angles between the double bond and the bond between unsaturated carbon and the carbon adjacent to the unsaturated carbon were measured in both molecules, as seen in the Jmols of both structures. Significant differences were observed. The angle that was measured would be ideally be 120° in a unstrained alkene with sp2 hybridised carbon however, in the mono-hydrogenated, products (3) & (4) have slightly strained bonds. The bond angle in (3) was measured as 107.7° while the bond angle in (4) was measured as 112.4°. Since the difference between the unstrained bond angle and the bond angle in structure (4) is greater than that between the unstrained bond and the angle in structure (3). A greater deviation results in a greater strain hence explaining why structure (4) is more stable than structure (3).
The reactivity of different atropisomers of taxol intermediates
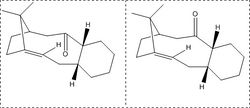
The intermediate for taxol has two main atropisomers one where the carbonyl points up and one where the moiety points down. When left alone, the compound remains in one isomer however in higher temperatures and pressures this intermediate interconverts between the atropisomers.
There are many possible conformers for this intermediate, four are in the table and the two hydrogenated chair structures are also displayed in the table. From this we can see that the intermediate molecules with the carbonyl group pointing up is more energetically unstable than the down pointing carbonyl atropisomers. The up-facing carbonyl atropisomer is 6.89kcal mol-1 and 5.38kcal mol-1 more stable than the down-facing carbonyl atropisomer for the chair and twist conformers. The main cause for this difference in this energy is due to torsional strain. The torsional strain in the upward facing carbonyls is caused by the repulsive effects by the nearby methyl group. In addition to this (MMFF94)calculations were done on the chair atropisomers. This consolidated the idea that the downward facing carbonyl conformers (60.031 kcalmol-1) were indeed more stable than there upward counterparts (70.5284 kcalmol-1.)
| Configuration | Carbonyl Up Chair | Carbonyl Up Twist | Carbonyl Down Chair | Carbonyl Down Twist | Carbonyl Up Chair Hydrogenated | Carbonyl Down Chair Hydrogenated |
|---|---|---|---|---|---|---|
| Stretch | 2.6695 | 3.1411 | 2.4470 | 2.5578 | 2.9022 | 2.9660 |
| Bend | 15.8760 | 15.8535 | 11.9913 | 12.7038 | 17.1628 | 13.6042 |
| Stretch-bend | 0.3932 | 0.3775 | 0.2048 | 0.2649 | 0.6585 | 0.6171 |
| Torsion | 18.2459 | 19.9800 | 16.2542 | 19.1724 | 22.9239 | 21.5659 |
| Non-1,4 VDW | -1.1264 | -0.1885 | -1.2688 | -0.6287 | -1.0806 | -0.8787 |
| 1,4 VDW | 12.6834 | 13.5548 | 12.1058 | 13.3374 | 15.7462 | 15.9144 |
| Dipole/dipole | -0.1463 | -0.0260 | 0.2600 | 0.2845 | -2.0321 | 0.0000 |
| Total | 48.8878 | 53.0694 | 41.9943 | 47.6921 | 58.3130 | 53.7888 |
The hydrogenated versions of the chair intermediates show an increase in energy compared to their original unsaturated intermediate. The bond strain is far greater thus increasing the energy of the molecule. This destabilisation is known as olefin strain [2] and explains the molecules resistance to hydrogenation.
Semi-empirical Molecular orbital theory
The molecular orbitals of a chloro dialkene
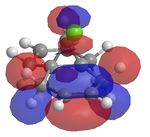
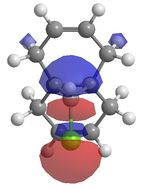
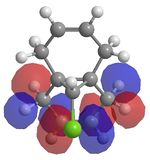
It has been shown that chlorocarbenes, a very reactive electrophilic reagent, reacts with alkenes, regioselectively, to yield the a cyclopropane. To understand why this regioselectively occurs calculations using MOs were done. Molecule 12 which is illustrated in the Jmol below was optimised and lowered in energy using MM2 and then loecular orbitals were calculated using MOPAC under a RM1 method, since the PM6 method, did not yield the correct MOs. The diagrams shows the exo π orbital being stabilised and therefore less nucleophilic than before and more importantly less nucleophilic than the endo π orbital. This means that nucleophilic attack occurs at the endo π orbital.
Vibrational energies were taken of molecule 12 and a hydrogenated structure of 12 at the exo bond. Thus providing values if there was no C-Cl interations. The C-Cl stretch occurs at a lower wave number in molecule 12 (770.86cm-1) compared to its hydrogenated counterpart (780.03cm-1). This can be explained by the electron donation from the exo π orbital into the σ*. The endo C=C stretch however is higher in energy than the same stretch in the hydrogenated molecule. This supports the theory above. So the endo C=C bond is more nucleophilic than the exo C=C bond due to the electronic interactions between the C-Cl σ* orbital interacting with the exo C=C bond π orbital, making this bond more diffuse and lower in energy. As a result electrophilic addition occurs on the endo part of the molecule.
| Molecule Orbital | HOMO -1 | HOMO | LUMO | LUMO +1 | LUMO +2 |
|---|---|---|---|---|---|
| Gauss | 
|
|
|

|
|

| Bond | Molecule 12 | Mono Hydrogenated Molecule 12 (exo to chlorine) | ||||
| Calculated Bond Length/Å | Wavenumber of stretch/cm-1 | Vibration | Calculated Bond Length/Å | Wavenumber of stretch /cm-1 | Vibration | |
| C-Cl | 1.760 | 770.86 |  |
1.760 | 780.03 |  |
| C=Cendo | 1.340 | 1757.34 | 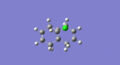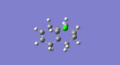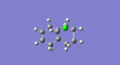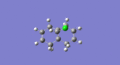 |
1.40 | 1753.72 |  |
| C=Cexo | 1.340 | 1737.03 |  |
N/A (C-C bond =1.54) | N/A | N/A |
Monosaccharide chemistry: glycosidation
| Molecule | A | A\' | B | B\' | C | C\' | D | D\' |
|---|---|---|---|---|---|---|---|---|
| Stretch | 2.6573 | 2.5566 | 2.7937 | 2.6642 | 1.9356 | 2.0169 | 1.8287 | 1.8944 |
| Bend | 12.5409 | 13.6377 | 10.6328 | 10.2950 | 13.1059 | 13.5489 | 14.9024 | 16.2794 |
| Stretch-bend | 1.0944 | 1.1015 | 0.9885 | 0.9666 | 0.6585 | 0.7394 | 0.7315 | 0.7656 |
| Torsion | 3.7359 | 1.7749 | 2.6855 | 2.8483 | 8.4649 | 9.9799 | 7.8714 | 7.3886 |
| Non-1,4 VDW | -0.4596 | -3.0814 | 0.5452 | 0.6319 | -2.6700 | -3.1337 | -3.7678 | -2.9970 |
| 1,4 VDW | 18.9370 | 18.7914 | 18.2740 | 18.9983 | 17.9663 | 17.9823 | 17.2593 | 16.8243 |
| Charge/Dipole | -18.0654 | 10.9121 | -19.4317 | -24.7811 | -9.9299 | -6.8698 | -5.1749 | -5.5737 |
| Dipole/dipole | 6.1143 | 4.4260 | 5.5848 | 5.6257 | -2.6959 | -2.2847 | -1.5974 | -1.9513 |
| Total | 26.5547 | 49.4764 | 22.5903 | 17.2489 | 26.8355 | 31.9791 | 32.0532 | 32.6303 |
| PM6 | -91.17760 | -91.66266 | -88.72610 | -88.72816 | -91.65888 | -91.64955 | -90.51145 | -90.51321 |
References
- ↑ Holger Braunschweig, Manuela Gross, Mario Kraft, Marc O. Kristen, and Dirk Leusser, J. Am. Chem. Soc. 2005, 127(10), 3282–3283 DOI:10.1021/ja042408s
- ↑ Philip M. Warner, Chem. Rev., 1989, 89 (5), 1067-1093, DOI: 10.1021/cr00095a007
