Rep:Mod:parkdara
MODULE 1: The Basic Techniques of Molecular Mechanics and Semi-empirical Molecular Orbital Methods for Structural and Spectroscopic Evaluations
Modelling Using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
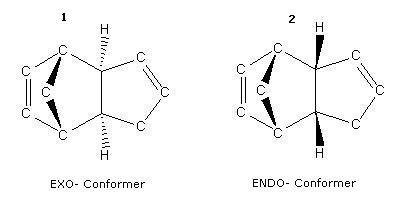
Cyclopentadiene readily dimerises at room temperature. The structure of the resultant dimer can be one of two possible conformational isomers:-
| 2D Representation | 3D Representation | |
|
 |
|
| 1 - "Exo" Product | 2 - "Endo" Product | |
Experimentally, it is observed that in fact the endo- conformer (2) is preferred over the exo- conformer (1).
| Molecule | 1 | 2 |
 |
| |
| Total Energy (kcalmol-1) | 20.0910 | 23.0455 |
Analysis of both conformers as to their total energies yields the following figures on the right:-
Under thermodynamic control, it would be observed that the conformer affording the lowest energy would predominate. Here, the thermodynamically preferred product would be the Exo- product, benefiting from a slight decrease in energy. However, the reaction is a (4+2) diels-alder, and is endo-selective on account of the "locked" cisoid-conformation of cyclopentadiene.
As such, the preferred endo- derivative indicates that the dimerisation of cyclopentadiene suffers kinetic control.
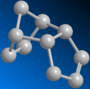
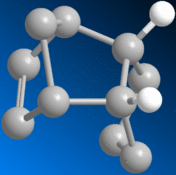
Further hydrogenation of the dimer can occur, forming the regioisomers 3 and 4:-
| 2D Representation | 3D Representation | |
|
 |
|
| 1 - "Exo" Product | 2 - "Endo" Product | |
By similar way of molecular mechanical comparison, the following values were yielded for the hydro-derivatives of dimer 2:-
| Molecule | 3 | 4 |
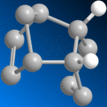 |
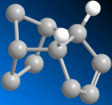
| |
| Stretch | 0.6396 | 0.4911 |
| Bend | 12.6446 | 9.8105 |
| Torsion | 5.6408 | 6.3952 |
| Van Der Waals | 1.9646 | 1.6261 |
| Total Energy (kcalmol-1) | 19.9699 | 17.7425 |
Conformationally, there is little spatial discrepancy between the two hydro- derivatives.
However, upon analysing the various values returned from MM2 analysis, it is apparent that 3 appears less strained.
The stretch and bend values are higher for 3 than they are for 4. These show the energy required to stretch and bend each respective molecule. Both are seen here to be higher for 3 than 4, implying that compound 3 is more structurally stable, as it takes more energy to disrupt it. The torsion value indicates the amount of torsional strain imposed on each molecule, which is, again, higher for 4 than 3.
This can be rationalised by the fact that the double bond is situated on the 6-membered ring on 3, whereas it is situated on the 5-membered ring on 4. Since the double bond affords additional strength over single bonds, the amount of flexibility within the bond is much less. The amount of torsional strain introduced in a 5-member ring is more than that in a 6-membered ring. Thus, molecule 3 can be said to be under less strain than 4.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
Heteroatomics and their derivatives have the added tendency to succumb to nucleophilic attack due to the electron-withdrawing, and thus destabilising heteroatom within the aromatic framework.
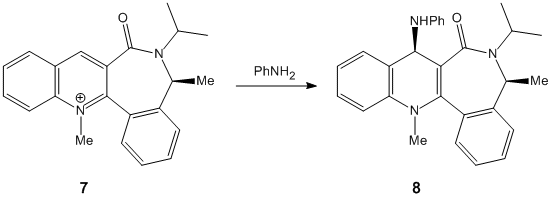
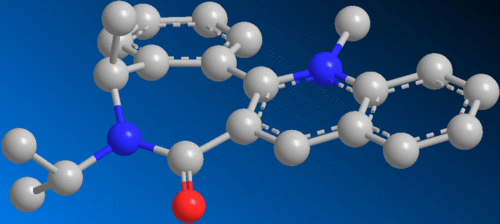
Prolinol, a pyridine analogue, readily undergoes para-directed nucleophilic attack from Grignard-activated species:-
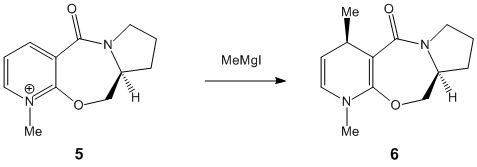 |
5 |
| |
 |
7 |
|
The stereochemistry resulting from the attack may be gauged from the preferred conformation of the reacting molecules and understanding the influence of steric hindrance.
Application of the MM2 force field yields the following values for reactants 5 and 7:-
| Molecule | 5 | 7 |
 |

| |
| Stretch | 1.0249 | 2.1640 |
| Bend | 10.6292 | 11.9287 |
| Torsion | 3.8952 | -3.1954 |
| Van Der Waals | 10.8233 | 21.1671 |
| Total Energy (kcalmol-1) | 30.5018 | 36.5563 |
It is observed that the closest possible sterically-hindering group in both reactant 5 and 7, the carbonyl, is found to lie beneath the plane of the aromatic ring. The carbonyl group is described as being "down" in this example. However, the meaning of down relates to the relative position of the carbonyl to the nearby benzene ring. Infact, the carbonyl is equatorial in relation to the plain in the board. If the carbonyl had been "up", then it would surely take up an axial position. Due to the inflexibility of the structure possessing several benzene rings, an axial carbonyl which is sp2 hybridised would be far more disruptive to the structure than the axial hydrogen.
Thus, the methyl group is directed into its repective stereochemical position by the "down" carbonyl.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
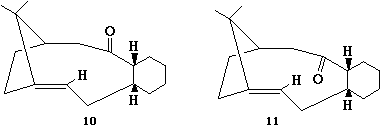
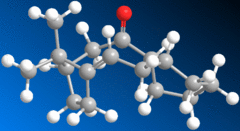
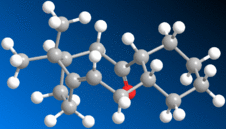
An example of atropisomerism, whereby two conformers switch between each other readily, may be found in the synthesis of Taxol, a cancer drug. This phenomenon is observed in the intermediate:-
| 2D Representation | 3D Representation | |
|
 |
|
| 10 - "Axial" Carbonyl | 11 - "Equatorial" Carbonyl | |
The carbonyl group is observed to alternate readily between the two positions shown. This atropisomerism is further accompanied by the fact that the alkene reacts rather slowly.
MM2 for the two intermediates yields:-
| Molecule | 10 | 11 |
| Stretch | 2.4697 | 2.6285 |
| Bend | 10.9258 | 10.7425 |
| Torsion | 17.2608 | 22.3014 |
| Van Der Waals | 12.4275 | 13.6942 |
| Total Energy (kcalmol-1) | 41.8255 | 48.2275 |
The result through optimisation of the structure for the two conformers 10 and 11 gave that overall, the two conformers are remarkably similar in terms of structural strain and thus energy. The results here indicate a slight advantage in 10, but I believe this to be slight imperfections on the part of the MM2 dynamics; the intermediates being gaudy cyclic structures that are rather conformationally flexible. From this, it would not be too unreasonable to envisage the aforementioned rapid interconversions between the two conformers, which are similar in energy.
The slow reductions of the alkene are due to a phenomenon known as "hyperstability"[1]. This arises in certain cyclic structures whereby the strain felt by the alkene in question is actually less than that felt by the corresponding alkane. In these cases, the alkene is said to be hyperstable and resistant to hydrogenation.
Modelling Using Semi-Empirical Molecular Orbital Theory
Regioselective Addition of Dichlorocarbene
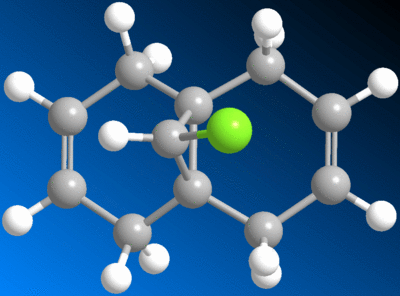 |
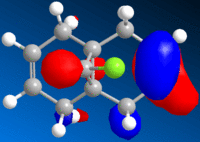
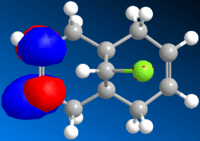
It was possible to view visualisations of the molecular orbitals of the compound:-
| Molecular Orbital | ||||
| HOMO-1 | HOMO | LUMO | LUMO-1 | LUMO-2 |
 |
 |
 |
 |
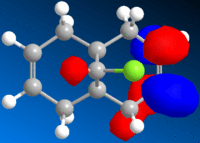
|
It is noted that the more vulnerable alkene of the two present is indicated by the manifestation of the HOMO of the molecule. The HOMO here is situated on the alkene group on the near side of the chlorine atom, and thus, the more vulnerable alkene group. The orbital extends below as can be seen in the HOMO diagram due to the steric hindrance from chlorine.
| IR Vibrations | ||
| Molecule | Di-12 | Mono-12 |
| C-Cl Stretch(cm-1) | 770.907 | 774.962 |
| C=C Stretch(cm-1) | (FAR)1737.1 | 1758.05 |
| (NEAR)1757.36 | ||
Both derivatives can been seen to contain their respective stretching characteristic of each functionality in the molecule.
In the di-unsaturated version, it can be seen that the nearer alkene group to the chlorine atom is skewed slightly higher in wavenumber and is down to influence from the bulky cholrine group.
Structure based Mini project using DFT-based Molecular orbital methods
Stereoselective dissolving metal reductions
The reduction of carbonyl and aromatic compounds can be facilitated by so-called "dissolving metal reductions", using a mixture of reagents such as Li/NH3 in tert-butoxide, generally known as a Birch Reduction.
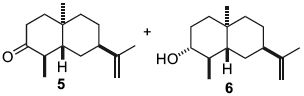
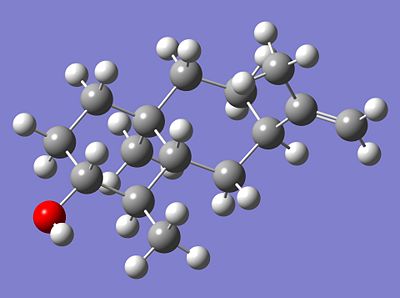
A recent natural product total synthesis was attempted, whereby a ketone (5) was reduced via Birch Reduction and gave a highly stereoselective ratio of an alcohol (6).
| 2D Representation | 3D Representation | |
|
| |
| Alcohol 6 | ||
By means of DFT Geometry Optimisation and other branching techniques, the geometry and various spectroscopic and physical data can be emulated.
13C NMR Prediction
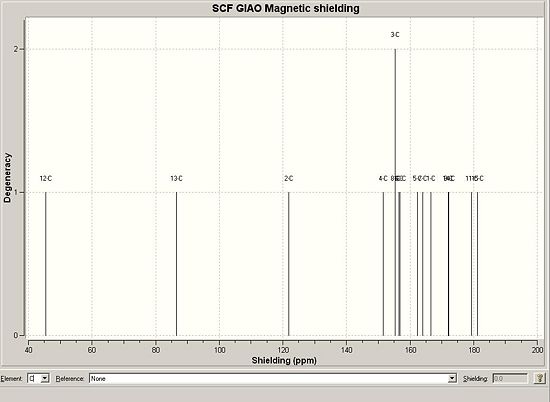
The 13C NMR Prediction was made using the GIAO approach, which uses quantum mechanical density functional theory.
The following was returned from the approximation:-
| Literature 13C NMR Data | |
| PPM | -C |
| 14.87 | 14 |
| 16.68 | 15 |
| 22.82 | 12 |
| 23.08 | 8 |
| 26.05 | 6 |
| 30.92 | 2 |
| 33.78 | 10 |
| 37.10 | 9 |
| 38.88 | 4 |
| 39.19 | 7 |
| 39.93 | 1 |
| 43.22 | 5 |
| 76.86 | 3 |
| 110.67 | 13 |
| 147.11 | 11 |
|
| 13C NMR Data | ||
| PPM | Degeneracy | -C |
| 45.4848 | 1 | 12 |
| 86.6293 | 1 | 13 |
| 121.885 | 1 | 2 |
| 151.591 | 1 | 4 |
| 155.298 | 2 | 3 |
| 156.438 | 1 | 6 |
| 156.855 | 1 | 10 |
| 162.383 | 1 | 5 |
| 164.054 | 1 | 7 |
| 166.563 | 1 | 1 |
| 171.976 | 1 | 9 |
| 172.23 | 1 | 14 |
| 179.385 | 1 | 11 |
| 181.27 | 1 | 15 |
The NMR returned varied to some degree from the literature values.
Vibrational Analysis (IR)
| IR Vibrations | ||
| Stretch Type | Frequency (cm-1) | |
| O-H (Alcohol) Stretch | 3833.58 | |
| C=C (Alkene) Stretch | 1717.19 | |
The characteristic stretches of the alcohol and the alkene are present.
Circular Dichroism (CD) Analysis
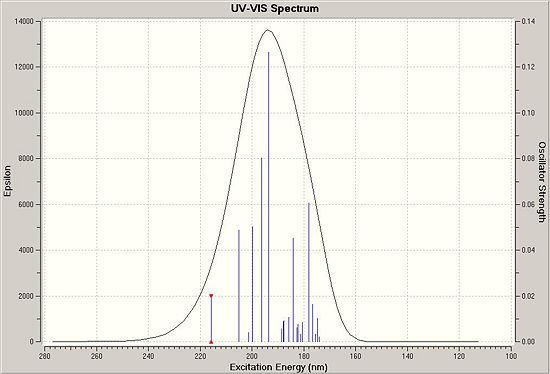
|
- ↑ Evaluation and prediction of the stability of bridgehead olefins DOI: 10.1021/ja00398a003
