Rep:Mod:organic2124
Organic Module
The Hydrogenation of Cyclopentadiene Dimer
The stuctures of the molecules used in this part of the module are shown below:
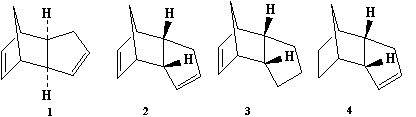
When cyclopentadiene dimerises there are two different forms (stereoisomers) that it is able to take; these are the endo- or exo- forms. Specifically endo-dicyclopentadiene has both the hydrogens pointing up, shown in figure 1, and exo-dicyclopentadiene has both the hydrogens pointing down, figure 2. One of the structures if more stable than the other, and therefore using molecular mechanics, the most stable form could be figured out, as well as determining whether it was thermodynamically or kinetically controlled.

Using ChemBio3D both compound 1 and 2 were drawn out as shown in figures 1 and 2 shown above. Once these had been drawn out the molecular dynamics was run using the MM2 force field. The resulting energies of the two molecules are shown below:
| Molecule | Energy (kcal/mol) | Energy (kJ/mol) |
|---|---|---|
| 1 | 31.9 | 134 |
| 2 | 34.0 | 142 |
This shows that thermodynamically molecule 1 is more stable due to it being lower in energy; however, as the endo product is formed specifically in the reaction, i.e molecule 2, it must be kinetically controlled, confirming that the endo product has a more stable transition state than the exo product. This shows that the MM2 model is not sufficient to predict the most stable form as it cannot predict that the kinetic product will predominate when it does.
The same process was run for molecules 3 and 4 with them being drawn in ChemBio3D, shown as figures 3 and 4, and then the MM2 force field run. The results of these are shown in the table below. It also shows other variables such as stretching, bending, torsion, Van der Waals and hydrogen bonding.
The results of these are shown in the table below. It also shows other variables such as stretching, bending, torsion, Van der Waals and hydrogen bonding.
| Molecule 3 | Molecule 4 | |
|---|---|---|
| Energy (kcal/mol) | 35.7 | 31.2 |
| Energy (kJ/mol) | 149 | 131 |
| Stretching | 1.27 | 1.11 |
| Bending | 19.7 | 14.5 |
| Torsion | 10.9 | 12.5 |
| Van der Waals | 5.63 | 4.50 |
| Hydrogen Bonding | N/A | N/A |
From the results it can be seen that the thermodynamically more stable of the two hydrogenation products is molecule 4. From comparison of the different properties given it can be seen that the biggest change in the energy contribution comes from the bending value; where in molecule 3 it is 5.2 kcal/mol higher than that in molecule 4. The differences in the other values are so small that they can be neglected in this instance. The difference in energy of bending between 3 and 4 can be explained due to torsional strain within the ring in molecule 3. The dihedral angle is shown to be 106° in molecule 3 where in reality the angle should be 120°.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
Below is a picture showing the molecules that were used in this model:
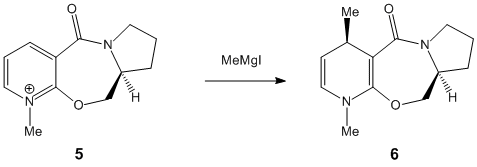
Again using ChemBio3D the reagents, molecules 5 were drawn out and represented in the programme as figures 5 and 6 respectively, shown below:
Figure 5: Molecule 5 Coplanar
Figure 6: Molecule 5 antiperiplanar to the chiral centre
Having set up the molecules, the geometry was optimized using the MM2 force field. Initially for molecule 5, there was an energy of 43.1 kcal/mol. When trying to run this when there was the MeMgI present produced an error message and so could not be run. The literature2 suggested thatt there were two different starting orientations possible for the carbonyl; these being the carbonyl group being above the plane of the pyridine, and the other stating that the carbonyl group would be antiperiplanar to the hydrogen at the chiral centre. The optimized geometry above, was for the coplanar carbonyl. When the geometry was optimized for the carbonyl group being anti periplanar to the hydrogen the energy given was 44.7 kcal/mol, slightly higher in energy. As the Mg co-ordinates with the Oxygen which is sitting either above the plane, or in the plane, the incoming methyl group will also attach at the top of the ring.
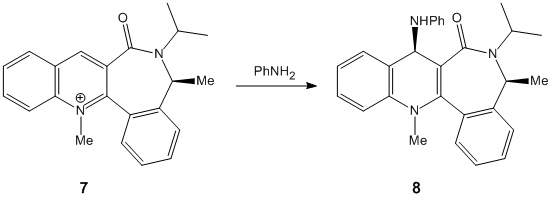
The molecules for 7 were then drawn again in ChemBio 3D and are shown to the left and right.
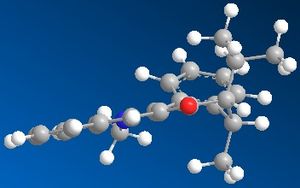
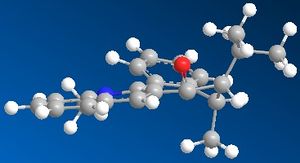
As can be seen, one form is where the carbonyl is below the plane of the pryridine ring; with the other form being in the plane of the ring. The product from this reaction can be rationalised due to knowledge of both Nitrogen and Oxygen. As they both contain lone pairs of electrons, there would be lone pair lone pair repulsions, which would direct the incoming nitrogen group away from the oxygen atom; hence onto the other side of the ring to the oxygen. You can also see that if the nitrogen group were to attack the same side as the oxygen there would be a great deal of steric hindrance which would also prevent the attack at this point. The energy of the molecule with the carbonyl group in the plane of the ring was given as 63.5 kcal/mol, and the energy with the molecule below the plane of the ring was 162 kcal/mol. This shows a huge difference in energy, however the nitrogen group will still not attack in the same plane as the carbonyl group due to the reasons outlined above.
Stereochemistry and reactivity of an Intermediate in the Synthesis of Taxol
Using ChemBio3D both of the Taxol intermediates were drawn. These are shown in the picture below and numbered 10 and 11:
Taxol molecule 10
Taxol molecule 11
Using the MM2 molecular dynamics was then run and the outcome was that molecule 10 had an overall energy of 54.6 kcal/mol, and molecule 11 had an overall energy of 128 kcal/mol, showing that 10 was extremely more thermodynamically stable than molecule 11; in fact, the energy in 11 is more than double that in 10. Molecule 10 has the twist boat form, which should be high in energy due to the strains on the ring. As molecule 10 has the lowest energy of the two, the molecule could be manually edited to create a chair chair conformation, which may result in lower energy. This was carried out and the energy value obtained was 44.4 kcal/mol, 10 kcal/mol lower in energy when having the 6 membered ring in a chair conformation; this molecule can be seen below:
Taxol in the Chair-Chair formation
The alkene reacts slowly at first due to it being a hyperstable alkene. This means that even when it undergoes reactions with strong catalysts, it has a tendency to not want to hydrogenate quickly, or even at all, due to the strain energy in the ring.
Modelling Using Semi-empirical Molecular Orbital Theory
The dialkene (figure 5) was set up using ChemBio3D and the MM2 molecular dynamics run which optimized the energy to 17.9kcal/mol. A MOPAC/PM6 was then set up and run which optimized the geometry even more to allow for the next part of the simulation. After this had been run the molecular orbitals were able to be shown. The pictures of these are shown below:
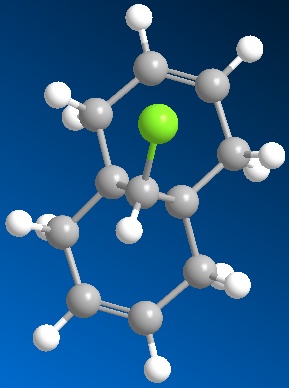
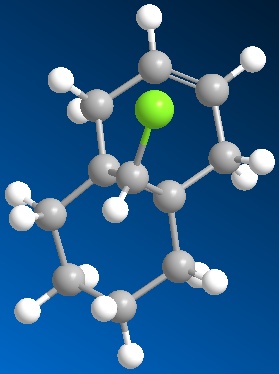




From inspection of the HOMO it is clear that the most nucleophilic of the two alkene bonds is the one that is present under the Cl atom. This is due to the fact that the majority of the charges are shown on this bond in the HOMO. The model, and the inspection of the different molecular orbitals show that it is possible to discriminate between the two different alkene bonds as there is a difference shown up in each of the pictures of the molecular orbitals.
Once this had been run, a monoalkene model was created for comparison with the dialkene. This can be seen in figure 6. The models were sent to SCAN to give an output of an IR spectrum for comparison. The different IR stretches can be seen in the tables below:
| Frequency (1/cm) | Functional Group |
|---|---|
| 3156 | C-H alkene under Cl |
| 3176 | C-H alkene not under Cl |
| 1757 | C=C under Cl |
| 1737 | C=C not under Cl |
| 770 | C-Cl |
| Frequency (1/cm) | Functional Group |
|---|---|
| 3175 | C-H alkene under Cl |
| 1753 | C=C under Cl |
| 779 | C-Cl |
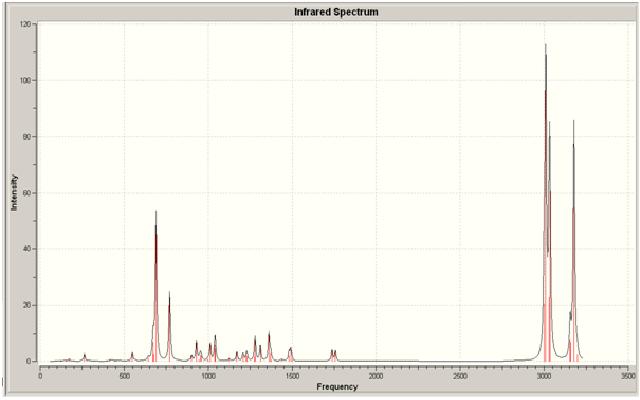
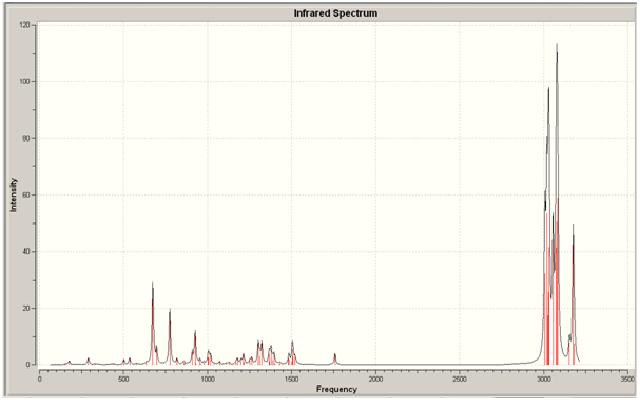
As can be seen from looking at the two molecules the IR stretches in each differ slightly. For one, there isn't a second alkene stretch in the monoalkene; which is correct as the second stretch should only be present in the dialkene. There is a slight difference in position in the spectra of the different peaks, which the monoalkene positions being lower for the C=C and higher for the C-Cl. This shows that the second alkene bond has an effect on the position in the spectra. it can also be seen that for the dialkene there are two stretches present for the C-H bonds on the alkenes; these are present at two different wavenumbers, which again shows the effect of the prescence of the Cl. There is only one C-H alkene stretch in the monoalkene.
The two spectra for both the dialkene and the monoalkene can be seen below:
Dialkene
Monoalkene
Structure based Mini project using DFT-based Molecular orbital methods
When searching literature for a project to work on I came across the paper entitled: 'Electrochemical carboxylation of N-(2-Bromopropionyl-4-R-phenyloxazoliadin-2-one); An efficient route to unsymmetrial methylmalonic ester derivatives". When looking further into this paper it was evident that within the reaction there were two diastereoisomers formed. The reaction scheme for this is shown below:

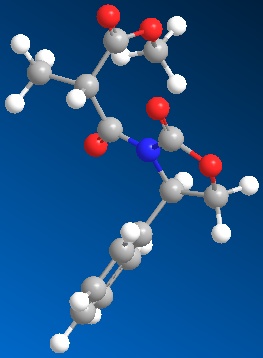
As before these molecules were drawn out in ChemBio 3D, shown in figures 7 and 8, and sent to SCAN to run an NMR, IR, CD and Optical Rotation analysis for comparison with the literature. The results of these different techniques are outlined below.

The IR assignments for both molecules are in the table below:
| Functional Group | Frequency Molecule 2a 1/cm | Frequency Molecule 2b 1/cm |
|---|---|---|
| C=O in 5 membered ring | 1883.94 | 1883.94 |
| C=O in ester | 1843.62 | 1840.41 |
| C=O inbetween the N and C | 1764.96 | 1762.99 |
| C-N | 1386.13 | 1385.82 |
| C-N | 1349.17 | 1350.47 |
| C-N | 1277.12 | 1277.96 |
| C-O | 1138.43 | 1139.03 |
| C-O | 1096.37 | 1095.90 |
| C=C aromatic | 1017.74 | 1017.77 |
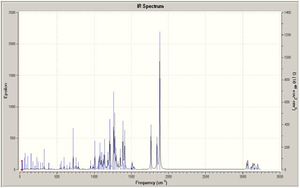
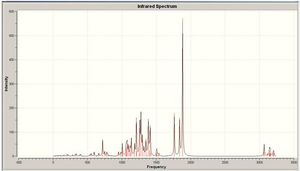
All values in the table above are given to 2dp to show the difference between the two molecules. As you can see, there is barely any difference between them, and so IR proves ineffective in trying to differentiate between them. This would be true as they are actually the same molecule, however they have the direction of the group orientated differently, therefore showing that the IR should be the same for both of the molecules. The IR spectra for both molecules are shown at the side of the page in figures 9 and 10.
The NMR assignments for both the molecules is shown below:
| Atom Number | 2a peak assignment | 2a Literature peak assignment | 2b Peak assignment | 2b Literature Peak assignment |
| 4 | 178.8 | 170.8 | 179.7 | 170.7 |
| 10 | 146.2 | 153.7 | 146.1 | 153.7 |
| 2 | 137.0 | 138.8 | 137.6 | 138.4 |
| 3 | 126.9 | 125.9 | 126.9 | 125.8 |
| 15 | 68.7 | 70.2 | 70.3 | 70.3 |
| 7 | 61.4 | 57.8 | 61.9 | 57.9 |
| 8 | 51.3 | 52.5 | 50.6 | 52.4 |
| 5 | 48.6 | 45.7 | 48.6 | 45.6 |
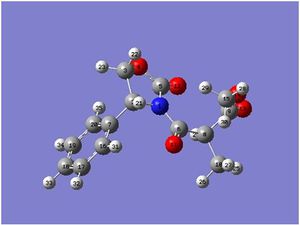
The peaks are assigned according to the numbered molecules as shown in the thumbnails figures 11 and 12, and the NMR for both molecule 2a and 2b can be seen in figures 13 and 14. The figures match up with those given in literatue, however again the two molecules are too similar to be able to differentiate between them, and so this is not the best way to distinguish between them.



Next a UV visible spectrum was run for both of the molecules. This again proved that the maximum absorption for both 2a and 2b was at around 190 nm; again proving that this technique was not useful in identifying the individual molecules.
The final analysis technique which was run was the Optical Rotation. The expected output for this was that one of the diasteroisomer had a positive value and the other had a negative value. When the results came through this proved right, with molecule 2a having a value of -238 degrees, and molecule 2b having a value of +210 degrees. Therefore what can be concluded is that the best analytical technique to distinguish between the molecules is to measure the optical rotation of each.
References
- A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- P. Camps, F. Perez and S. Vazquez, Tetrahedron, 1997, 53, 28. doi:10.1016/S0040-4020(97)00595-4
- M. Feroci, A. Inesi, M. Orsini and L. Palombi,Organic Letters, 2002, 4, 16. DOI:10.1021/ol025939z