Rep:Mod:oop17
Molecular Modelling 2 - Oana Popescu
NH3 Molecule
Molecule Type: NH3
Calculation Method: RB3LYP
Basis Set: 6-31G(D,P)
Final Energy (au): -56.55776873
Point Group: None
Item Table:
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986286D-10
Optimization completed.
-- Stationary point found.
JMol Simulation:
NH3 molecule |
The above optimisation file is linked here.
Vibrations:
Questions
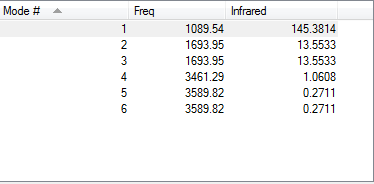
how many modes do you expect from the 3N-6 rule?
The molecule has 4 atoms; thus, (3*4)-6 is 6 vibrational modes, in line with results.
which modes are degenerate (ie have the same energy)?
Modes 2-3 and 5-6 respectively, in the above image.
which modes are "bending" vibrations and which are "bond stretch" vibrations?
1,2,3 - bend, 4, 5, 6 - stretch
which mode is highly symmetric?
Mode 4 (symmetrical stretches in all directions)
one mode is known as the "umbrella" mode, which one is this?
Mode 1 - symmetrical bend of all 3 molecules
how many bands would you expect to see in an experimental spectrum of gaseous ammonia?
2 Bands - Due to the degeneracy of results 2 and 3, and the last 3 results being too small to record on an infrared spectrum.
Charges
Expectation: The H-atoms are positive and the N-atom is negative, due to the high electronegativity of Nitrogen and the Electropositivity of Hydrogen.
Calculated Charges: Hydrogen: 0.375; Nitrogen: -1.125
N2 Molecule
Molecule Type: N2
Calculation Method: RB3LYP
Basis Set: 6-31G(D,P)
Final Energy (au): -109.52412868
Point Group: None
Item Table:
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401066D-13
Optimization completed.
-- Stationary point found.
JMol Simulation:
N2 molecule |
The above optimisation file is linked here.
Vibrations: One vibration at frequency 2457.33, infrared 0
Charges: Molecule is 100% covalent. No dipole found.
H2 Molecule
Molecule Type: H2
Calculation Method: RB3LYP
Basis Set: 6-31G(D,P)
Final Energy (au): -1.17853936
Point Group: None
Item Table:
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
JMol Simulation:
H2 molecule |
The above optimisation file is linked here.
Vibrations: One vibration at frequency 4465.68, infrared 0
Charges: Molecule is 100% covalent. No dipole found.
Reaction Energy Calculations
E(NH3)= -56.55776873au
2*E(NH3)= -113.11553746au
E(N2)= -109.52412868au
E(H2)= -1.17853936au
3*E(H2)= -3.53561808au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907au = -146.48kJ/mol
Since the energy change is negative, the reaction is exothermic; thus, the products have a lower energy than the reactants, and are more stable.
Molecule of Choice: Methane (CH4)
Molecule Type: CH4
Calculation Method: RB3LYP
Basis Set: 6-31G(D,P)
Final Energy (au): -40.52401404
Point Group: None
Item Table:
Item Value Threshold Converged? Maximum Force 0.000063 0.000450 YES RMS Force 0.000034 0.000300 YES Maximum Displacement 0.000179 0.001800 YES RMS Displacement 0.000095 0.001200 YES Predicted change in Energy=-2.256034D-08 Optimization completed. -- Stationary point found.
JMol Simulation:
CH4 molecule |
The above optimisation file is linked here.
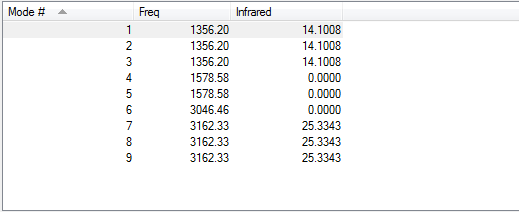
The Molecule has 9 vibrations 4 stretches (6-9) and 5 bends (1-5); 6 show a change in dipole moment and are infrared-active, whilst the other three are symmetrical from a dipole perspective and are not visible on an IR spectrum. Of the ones that are IR-active, bends 1-3 and stretches 7-9 vibrate at the same frequency; thus, they would show up as one band each on an IR spectrum, creating a final spectrum with 2 bands.
Charges:
The molecule itself isn't charged, but has a slight dipole, with the carbon being slightly electronegative at -0.930, and the hydrogens all slightly electropositive at 0.233.
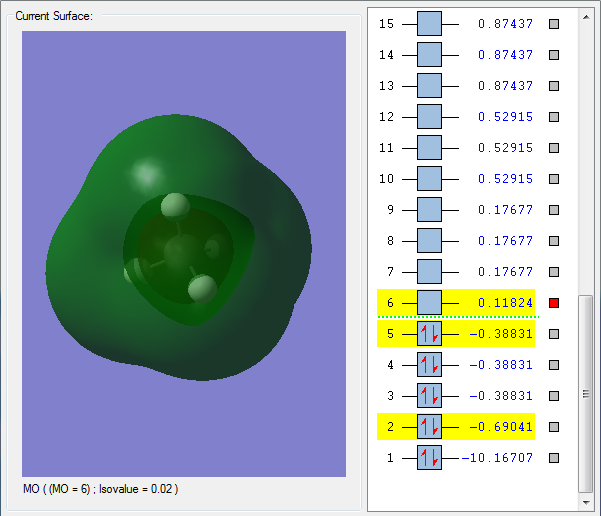
Some Molecular Orbitals
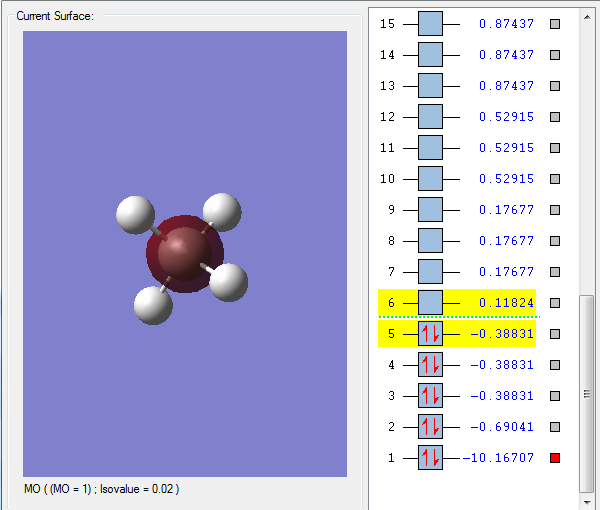
Orbital 1 - A low orbital that is very deep in energy; it stays close to the carbon and does not appear to be involved in bonding, most likely being the Carbon 1s orbital.
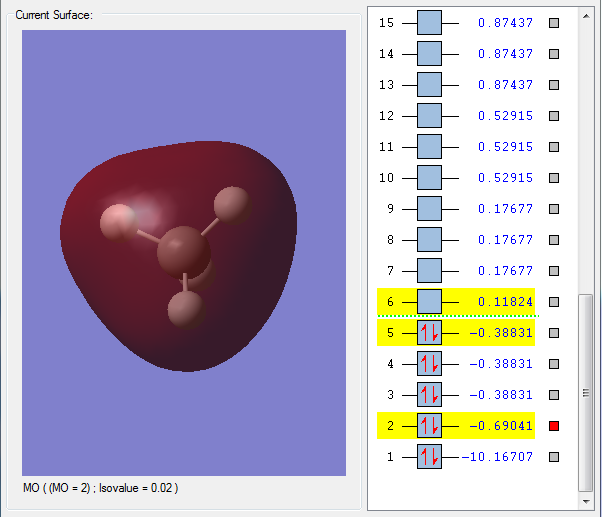
Orbital 2 - A lot higher in energy than the previous orbital, this encompasses both the Carbon and the Hydrogens, and is thus the first carbon-hydrogen bonding obrital, composed of the 2s orbital from Carbon and 1s orbital from Hydrogen, forming a sigma s-s bond. This can be seen in its round shape.
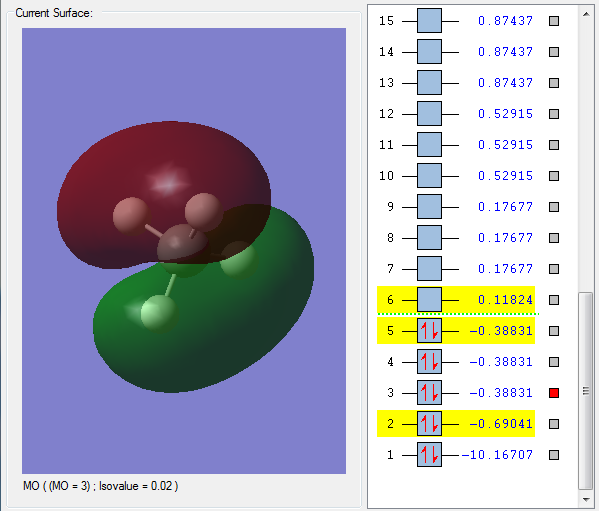
Orbital 3 - Another Carbon-Hydrogen Bonding Orbital, it consists of one of the Carbon p-orbitals and the Hydrogen 1s orbital; this can be seen from the 2 major "lobes" of the orbital, which suggest a p-orbital being involed.
Orbital 4 - This is the HOMO; similar to the previous MO, it is another S-P bond between Carbon and Hydrogen, using a different pi orbital from the carbon.
Orbital 5 - This is the LUMO; as seen in the picture, it has a different structure within the outside orbital, and thus appears to be an antibonding orbital. It is most likely the antibonding orbital of the aforementioned s-s bonding orbital, due to its round structure which does not suggest p-orbitals being involved.