Rep:Mod:og31
Transition States and Reactivity
Introduction
The potential energy surface (PES) of a molecule is a graph created from all the possible structures that a particular molecule could have.[1] The hills have high energy and so the molecule is likely not to stay in that conformation whereas the troughs have low energy so those structures are more stable. A transition state is a point on the PES that has a the highest energy along a reaction pathway and a minimum has the lowest energy. The differential of a transition state and minimum is 0 but the second differential is negative for a transition state and positive for minimum; this describes the curvature. By performing a frequency calculation, it is possible to tell if the structure is at a transition state because the calculation will have one negative frequency. The calculations performed were achieved using density-functional theory (DTF) and PM6 methods.
Nf710 (talk) 22:26, 17 November 2016 (UTC) It is an imaginary frequency, you could have given the relationship between the negative curvature and the imaginary frequency.
Reaction of Butadiene with Ethylene
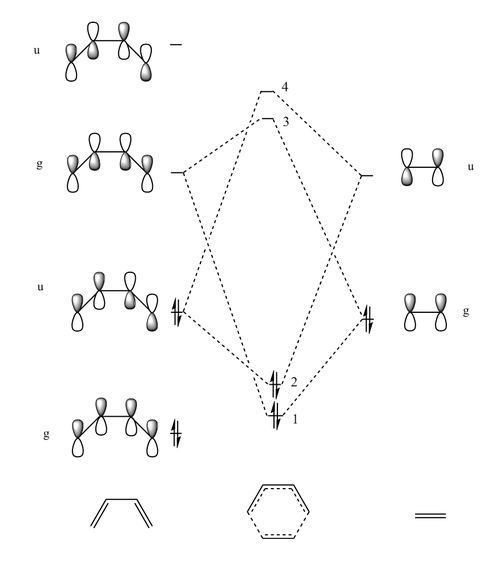
The Diels-Alder reaction between butadiene and ethylene was investigated. The frontier orbital MO diagram of the transition state between the two reactants can be seen in Figure 1 and the HOMO and LUMO of the reactants can be seen in Figures 2,3,4 and 5. These orbitals can be combined in the way shown in Figure 1 to produce the MOs seen in Figures 6,7,8 and 9. The orbitals can only combine if they have the same symmetry; an ungerade-ungerade interaction and gerade-gerade interaction will have an orbital overlap of non-zero. An example of a non-zero orbital overlap is when the HOMO of butadiene combines with the LUMO of ethylene, they both have ungerade symmetry. If two MOs with different symmetry try to combine the orbital overlap will be 0 i.e. non bonding.
 |
 |
 |
 |
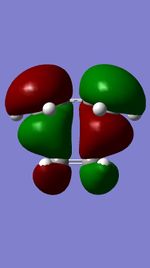 |
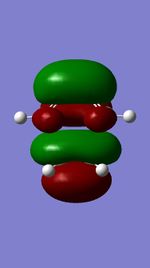 |
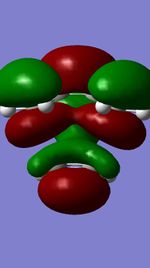 |
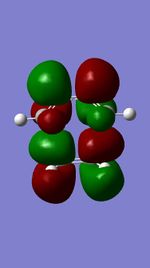 |
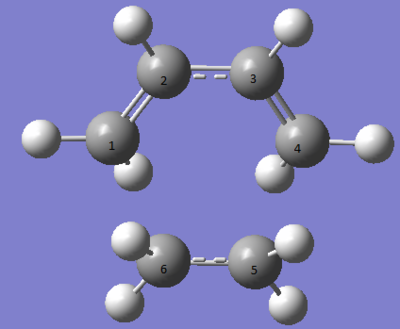
Table 1 shows that all of the bonds in the reactants, apart from the C-C bond in butadiene, get longer as the transition state is formed. This is expected because the double bonds are breaking and so they become more like single bonds which are longer, and the single bond in butadiene becomes a double bond so it gets shorter. Most of bond lengths in the transition state are between the lengths of an sp3 and sp2 carbon. [2] The calculated data matches the data that Goldstein et. al. found.[3] The Van Der Waals radius for a carbon atom is 170Å
[4] and the bond length of C4-C5 is smaller than that and can therefore be called a partial bond.
| Bond Lengths (Å) | Ref [3] | ||
|---|---|---|---|
| Reactants | Ethene | 1.33 | |
| Butadiene C-C | 1.47 | ||
| Butadiene C=C | 1.34 | ||
| Transition State | C1-C2 | 1.38 | 1.383 |
| C2-C3 | 1.41 | 1.407 | |
| C3-C4 | 1.38 | 1.383 | |
| C4-C5 | 2.27 | 2.273 | |
| C5-C6 | 1.39 | ||
| C6-C1 | 2.27 | 2.273 | |
| Products | C1-C2 | 1.51 | |
| C2-C3 | 1.32 | ||
| C3-C4 | 1.51 | ||
| C4-C5 | 1.55 | ||
| C5-C6 | 1.56 | ||
| C6-C1 | 1.55 |
GIF 1 shows the synchronous formation of the two bonds.
GIF 1: Transition state reaction path |
Nf710 (talk) 22:31, 17 November 2016 (UTC) Everything was well done in this section
Reaction of Benzoquinone with Cyclopentadiene
The next reaction that was computed was the Diels-Alder reaction between benzoquinone and cyclopentadiene. This reaction can yield an exo and an endo product and the energies of the reaction are tabulated in Table 2. The data shows that the energies of the products in both cases are more positive than the reactants which suggests that the reaction is endothermic. The thermodynamic product has the lowest Gibbs free energy and the kinetic product has the lowest activation barrier therefore the endo product is both the thermodynamic and the kinetic product which is confirmed by Tormena et. al.[5]
| Energy (kJ/mol) | ||
| Reactants | Cyclopentadiene | -509438.3563 |
|---|---|---|
| Benzoquinone | -1001357.467 | |
| exo | Transition state | -1510663.737 |
| Product | -1510782.895 | |
| Activation | 132.08629 | |
| Gibbs | 12.92796 | |
| endo | Transition state | -1510670.426 |
| Product | -1510783.837 | |
| Activation | 125.396515 | |
| Gibbs | 11.9854084 |
 |
 |
 |
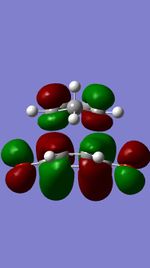 |
(At this angle it can be difficult to see what the difference is between the endo and exo TSs Tam10 (talk) 12:53, 9 November 2016 (UTC))
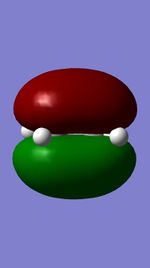
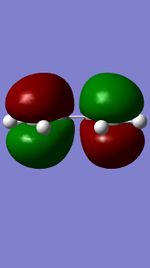
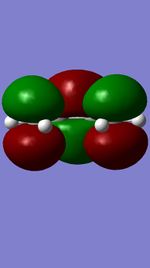
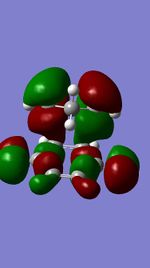
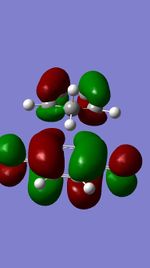
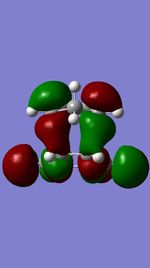
Figures 11,12,13, and 14 show the HOMOs and LUMOs of the exo and endo transition states. The nodes in the LUMOs and the orbital overlap in the HOMOs can be clearly seen between the two molecules.
Nf710 (talk) 22:48, 17 November 2016 (UTC) Nice uses of literature. 2 point you calculated your reactants from infinite separation, it should be from the first point on the irc optimized. you also didnt give any reason as to why the endo is the kinetic and thermo.
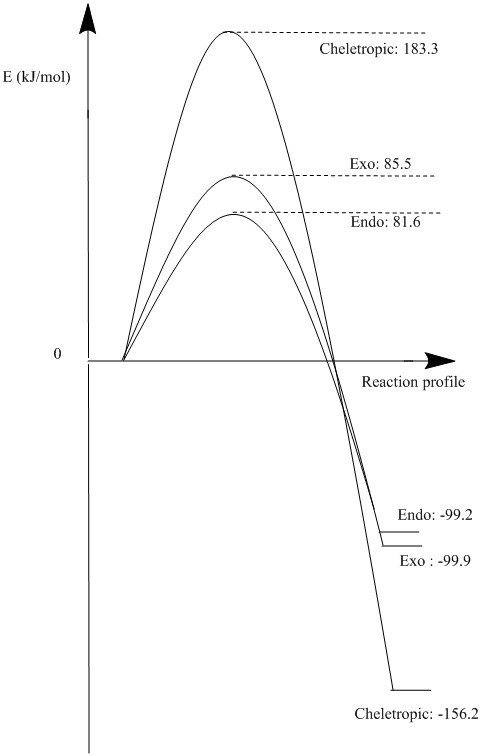
Diels-Alder vs Cheletropic
Xylylene can undergo a Diels-Alder reaction with sulphur dioxide to afford the exo and endo product however it can also undergo a cheletropic reaction to produce a different product. Table 3 shows the energies that have be computed for these reactions. Between the exo and endo products, the endo is the kinetic product and the exo is the thermodynamic product. The Cheletropic product is the most stable how it has the largest activation energy so it will only form under high temperatures. At room temperature, the Diels-Alder reaction will be more frequent and there will be be a mix of the endo and exo. It is likely that the endo product will be the major product because the energy difference of the reaction between the endo and exo is very small compared to the activation energy as seen in Figure 15. The IRC of the reactions can be seen in GIFs 2,3, and 4.
| Energy (kJ/mol) | ||
| Reactants | Xylylene | 467.630466 |
|---|---|---|
| Sulphur Dioxide | -311.4210807 | |
| exo | Transition state | 241.748182 |
| Product | 56.3301068 | |
| Activation | 85.538797 | |
| Gibbs | -99.87927861 | |
| endo | Transition state | 237.765298 |
| Product | 56.9838563 | |
| Activation | 81.5559127 | |
| Gibbs | -99.22552906 | |
| Cheletropic | Transition state | 339.534937 |
| Product | 0.005251 | |
| Activation | 183.325551 | |
| Gibbs | -156.2146364 |
 |
 |
 |
(What about the thermodynamic instability of xylylene? Tam10 (talk) 12:53, 9 November 2016 (UTC))
Conclusion
The exo and endo products of Diels-Alder reaction have different activation and Gibbs free energies and so there will be a major and a minor product when it is carried out experimentally. The data calculated from GaussView has been shown to correlate with the literature and give accurate bond lengths and energies.
References
- ↑ Silbey, Alberty, Bawendi. Physical Chemistry (4th ed.). Hoboken, NJ: John Wiley & Sons, 2005. (p.690-692)
- ↑ F. H. Allen , O. Kennard, D. G. Watson, L. Brammer, A. G, Orpen, and R. Taylor "Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds", J. Chem. Soc., Perkin Trans. 2, 1987, S1-S19.DOI:10.1039/P298700000S1
- ↑ 3.0 3.1 E. Goldstein , B. Beno, and K. N. Houk "Density Functional Theory Prediction of the Relative Energies and Isotope Effects for the Concerted and Stepwise Mechanisms of the Diels-Alder Reaction of Butadiene and Ethylene", J. Am. Chem. Soc., 1996, 118, 6036-6043.DOI:10.1021/ja9601494
- ↑ S. S. Batsanov "Van der Waals Radii of Elements", Inorg. Mater.,2001, 37, 9, 871-885DOI:10.1023/A:1011625728803
- ↑ C. F. Tormena, V. Lacerda Jr., and K. T. de Oliveira "Revisiting the Stability of endo/exo Diels-Alder Adducts between Cyclopentadiene and 1,4-benzoquinone", J. Braz. Chem. Soc., 2010, 21, 1, 112-118. DOI:10.1590/S0103-50532010000100017
