Rep:Mod:oIEETToE
NH3
| Summary | |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| E(RB3LYP) | -56.55776873 au |
| RMS gradient | 0.05399560 au |
| Point group | C3V |
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
N-H bond distance: 1.01798 Å
H-N-H bond angle: 105.741°
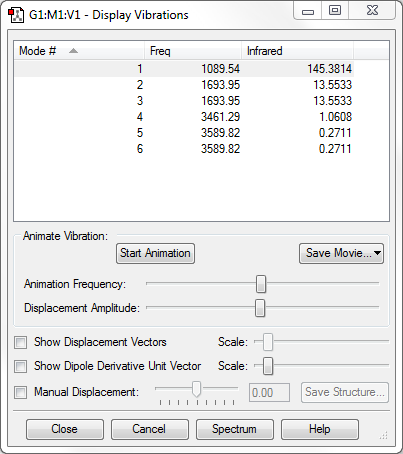
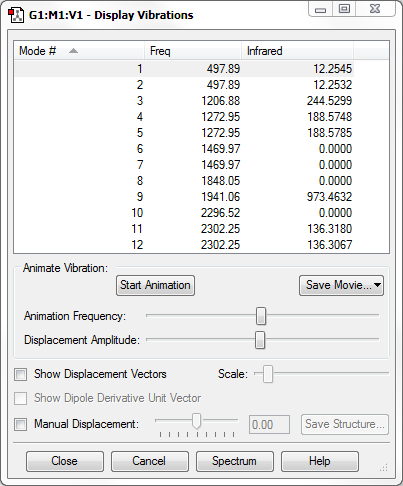
From the 3N-6 rule for non-linear molecules, we would expect 6 modes of vibration, which can be seen in the image above.
Modes 2 and 3 are degenerate, as are modes 4 and 5.
Modes 1, 2 and 3 are bending vibrations while modes 4, 5 and 6 are stretching vibrations.
Mode 4 is highly symmetric.
Mode 1 may be called the 'umbrella' mode as it slightly resembles a flipping or opening and closing umbrella.
We would expect 4 bands in the IR spectrum for gaseous NH3 as it appears that every mode has a change in dipole moment, although 2 of them would be quite weak.
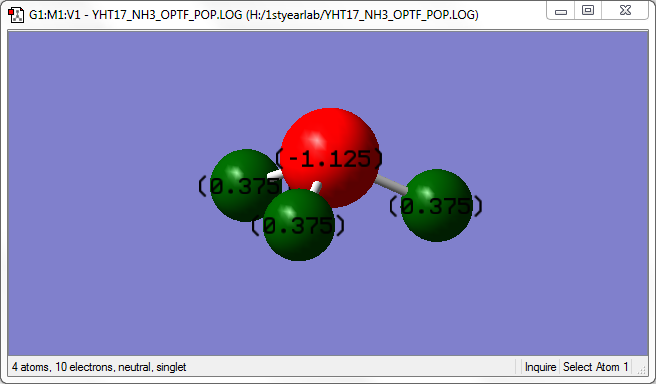
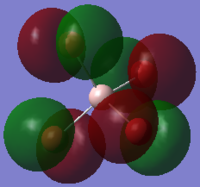
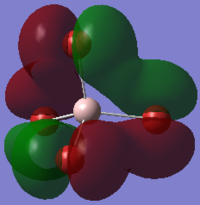
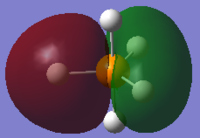
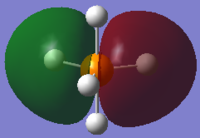
The image above shows the charges on each atom in NH3.
It is expected that N would have negative charge and H would have positive charge because N as a higher electronegativity than H.
The optimisation file for NH3 is linked to here
N2
| Summary | |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| E(RB3LYP) | -109.52412868 au |
| RMS gradient | 0.00000060 au |
| Point group | D∞H |
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
There is one mode of vibration at a frequency of 2457.33 (IR inactive).
The optimisation file for N2 is linked to here
H2
| Summary | |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| E(RB3LYP) | -1.17853936 au |
| RMS gradient | 0.00000017 au |
| Point group | D∞H |
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
There is one mode of vibration at a frequency of 4465.68 (IR inactive).
The optimisation file for H2 is linked to here
N2 + 3H2 -> 2NH3
| Energies | |
|---|---|
| E(NH3) | -56.55776873 au |
| 2×E(NH3) | -113.11553746 au |
| E(N2) | -109.52412868 au |
| E(H2) | -1.17853936 au |
| 3×E(H2) | -3.53561808 au |
| ΔE = 2×E(NH3)-(E(N2)+3×E(H2)) | -0.0557907 au = -146.48 kJ mol-1 |
This shows that the ammonia product is more stable than the gaseous reactants, which means the reaction is exothermic.
[AlBr4]-
| Summary | |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| E(RB3LYP) | -10529.67123680 au |
| RMS gradient | 0.00000140 au |
| Point group | Td |
Item Value Threshold Converged? Maximum Force 0.000003 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000021 0.001800 YES RMS Displacement 0.000011 0.001200 YES
Al-Br bond distance: 2.32607 Å
Br-Al-Br bond angle: 109.471°
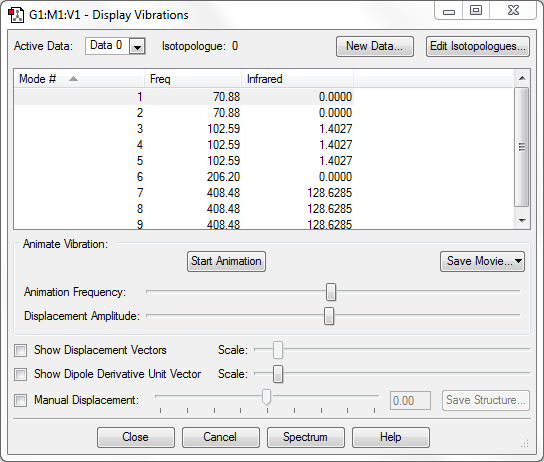
From the 3N-6 rule for non-linear molecules, we would expect 9 modes of vibration, which can be seen in the image above.
Modes 1 and 2 are degenerate, as are modes 3 to 5 and modes 7 to 9.
Because Br is not light compared to Al, only one mode, the highly symmetric mode 6, is a stretching vibration.
We would expect 2 bands in the IR spectrum for gaseous [AlBr4]- as there are two different frequencies at which there is IR absorbance (the others are IR inactive because there is no change in dipole moment). One of them would be quite weak.
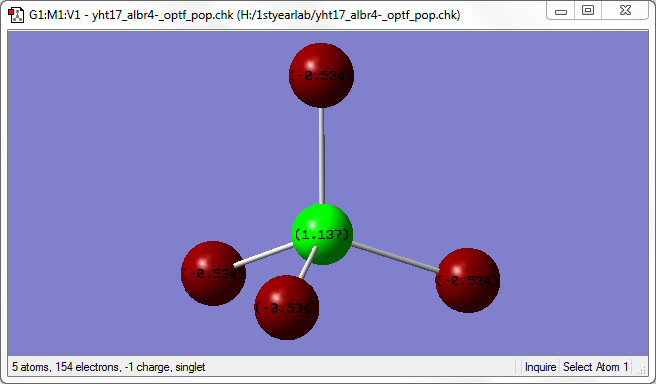
The image above shows the charges on each atom in [AlBr4]-.
It is expected that Br would have a lower charge than Al because Br as a higher electronegativity than Al.
The optimisation file for [AlBr4]- is linked to here
PH5
As we learn in the Periodicity course, this compound does not exist due to weak bonding between P and H caused by the size mismatch between the n=3 orbitals on the P and the 1s orbitals on the H. Therefore, all the information in this section is about a purely theoretical molecule and has little use.
| Summary | |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| E(RB3LYP) | -344.25491049 au |
| RMS gradient | 0.00000471 au |
| Point group | D3h |
Item Value Threshold Converged? Maximum Force 0.000009 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000055 0.001800 YES RMS Displacement 0.000022 0.001200 YES
P-H(axial) bond distance: 1.48687 Å
P-H(equatorial) bond distance: 1.43316 Å
H(axial)-P-H(axial) bond angle: 180.000°
H(axial)-P-H(equatorial) bond angle: 90.000°
H(equatorial)-P-H(equatorial) bond angle: 120.000°
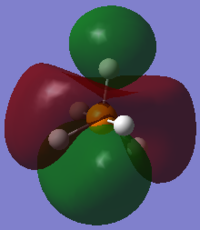
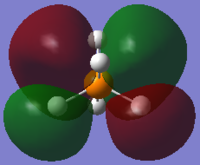
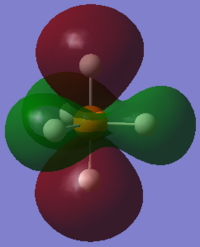
From the 3N-6 rule for non-linear molecules, we would expect 12 modes of vibration, which can be seen in the image above.
Modes 1 and 2 are degenerate, as are modes 4 and 5, modes 6 and 7 and modes 11 and 12.
Modes 1 to 7 may be considered bending vibrations while modes 8 to 12 may be considered stretching vibrations.
Mode 10 is highly symmetric.
We would expect 5 bands in the IR spectrum for gaseous PH5 as there are five different frequencies at which there is IR absorbance (the others are IR inactive because there is no change in dipole moment). One of them would be quite weak.
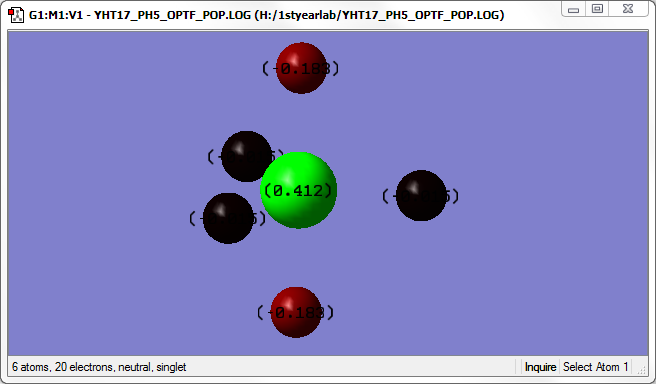
The image above shows the charges on each atom in PH5. The charge on each of the equatorial H atoms is not very visible, but they are -0.015.
P and H have similar electronegativity, so we would expect the charges to be quite balanced. However, there is some negative charge on the axial H atoms, perhaps so that the electrons can be further apart.
The optimisation file for PH5 is linked to here