Rep:Mod:mx4417
BH3
B3LYP/6-31G(d,p)
Frequency file:File:MX BH3 SYM OPT FREQ.LOG
Full mass-weighted force constant matrix:
Low frequencies --- -0.3987 -0.1915 -0.0053 25.8800 27.8261 27.8289
Low frequencies --- 1163.2000 1213.3197 1213.3224
Item Value Threshold Converged?
Maximum Force 0.000049 0.000450 YES
RMS Force 0.000024 0.000300 YES
Maximum Displacement 0.000193 0.001800 YES
RMS Displacement 0.000096 0.001200 YES
optimised BH3 molecule |
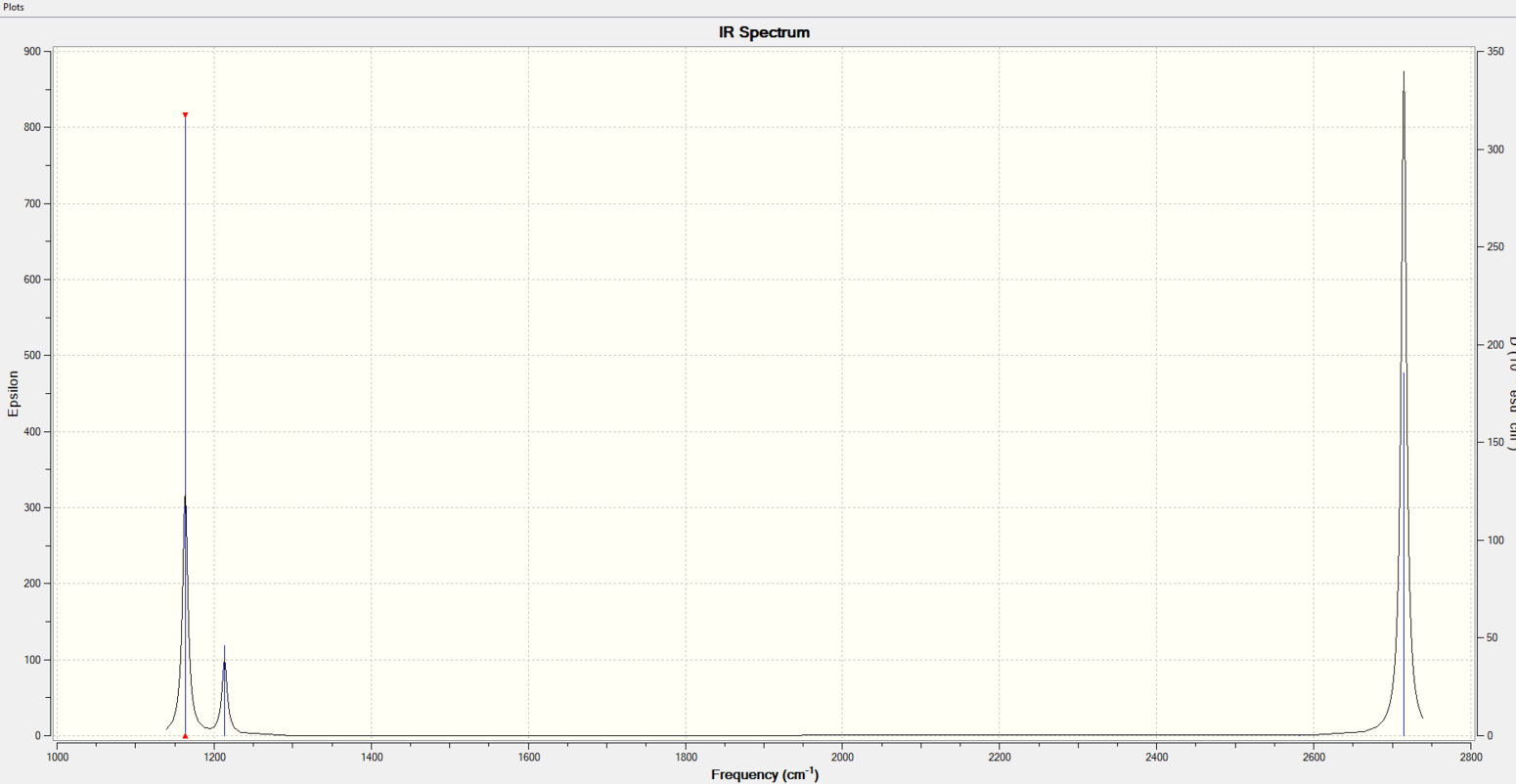
Vibrational spectrum for BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 93 | A2 | yes | out-of-plane bend |
| 1213 | 14 | E | very slight | bend |
| 1213 | 14 | E | very slight | bend |
| 2582 | 0 | A1 | no | symmetric stretch |
| 2715 | 126 | E | yes | asymmetric stretch |
| 2715 | 126 | E | yes | asymmetric stretch |
Only 3 peaks appears on the spectrum, as two bending vibration overlapped and had the same wavenumber and intensity. Also, for two asymmetric stretch, they overlapped completely. For the symmetric stretch, the dipole moment cancelled each other out, so it was IR inactive.
Good information for the vibrational analysis, you've identified the correct reasons for only 3 peaks being visible but your explanation could be improved by using terms like degeneracy, and the same intensity values won't affect the overlap. Smf115 (talk) 12:01, 18 May 2019 (BST)
The MO for BH3
The computed MO are very similar to the LCAOs model, which tells us LCAOs is a good approximation. The qualitative MO theory is very useful in most cases and provide a good approximation of the real MO.
Good inclusion of the calculated MOs onto the diagram, to improve your discussion could have been a bit more developed, for example considering some of the differences which you can see between some of the calculated and LCAO MOs. Smf115 (talk) 11:59, 18 May 2019 (BST)
The assocication energy
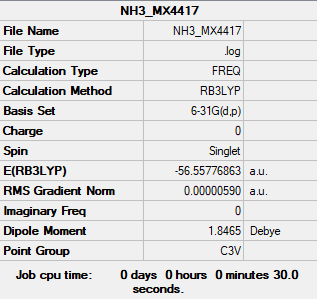
NH3
Low frequencies --- -8.4924 -8.4449 -0.0029 0.0336 0.1925 26.4139 Low frequencies --- 1089.7617 1694.1860 1694.1864
Frequency file:File:NH3 MX4417.LOG
Item Value Threshold Converged? Maximum Force 0.000009 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000040 0.001800 YES RMS Displacement 0.000017 0.001200 YES Predicted change in Energy=-3.940294D-10
optimised NH3 molecule |
NH3BH3
Frequency file:File:NH3BH3MX4417.LOG
Low frequencies --- -0.0615 -0.0457 -0.0067 21.6769 21.6828 40.5334 Low frequencies --- 266.0154 632.3610 640.1355
Item table:
Item Value Threshold Converged?
Maximum Force 0.000122 0.000450 YES
RMS Force 0.000059 0.000300 YES
Maximum Displacement 0.000621 0.001800 YES
RMS Displacement 0.000329 0.001200 YES
Predicted change in Energy=-1.786729D-07
Optimization completed.
optimised NH3BH3 molecule |
E(NH3)=-56.55777 a.u
E(BH3)=-26.61532 a.u
E(NH3BH3)= -83.22469 a.u
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]=-0.05160 a.u=-135.47 kJ/mol The B-N dative bond are relative weak compare to the B-N literature value -377.9 kJ/mol.
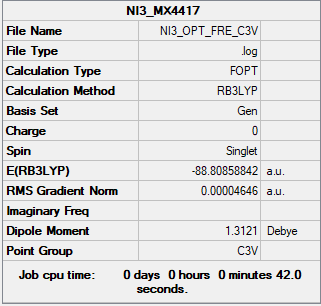
NI3
B3LYP/6-31G(d,p)LANL2DZ NI3
Frequency file:File:NI3 OPT FRE.LOG
Low frequencies ----2365.8802-1156.2248-1156.2227 -971.3056 -363.7620 -363.7518
Low frequencies --- 0.0087 0.0149 0.0193
Item Value Threshold Converged?
Maximum Force 0.000069 0.000450 YES
RMS Force 0.000030 0.000300 YES
Maximum Displacement 0.000116 0.001800 YES
RMS Displacement 0.000087 0.001200 YES
Predicted change in Energy=-1.767834D-09
Optimization completed.
optimised NI3 molecule |
N-I distance= 2.18411 A
In general, good structure information and correct implementation of the pseudopotential for NI3 in the Optimisation summary table, however, this should be from a Frequency calculation. The submitted file was also an Opt Freq calculation (as were your NH3 and NH3BH3) and not just a Frequency log file, and there was no pseudopotential implemented, you had used just the 6-31G(d,p) basis set. Smf115 (talk) 11:56, 18 May 2019 (BST)
Mini Project
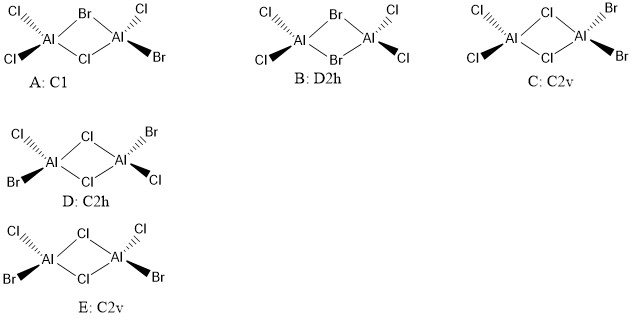
Five possible isomers
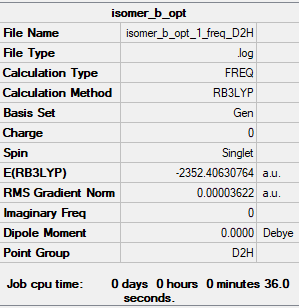
Isomer B (2 bridging Br ions)
Frequency file:File:ISOMER B OPT 1 FREQ.LOG
Low frequencies --- -4.8552 -4.8404 -3.5999 0.0035 0.0036 0.0044 Low frequencies --- 14.9772 63.3729 86.1222
Item Value Threshold Converged? Maximum Force 0.000080 0.000450 YES RMS Force 0.000036 0.000300 YES Maximum Displacement 0.001480 0.001800 YES RMS Displacement 0.000925 0.001200 YES Predicted change in Energy=-3.433146D-07 Optimization completed.
optimised isomer b molecule |
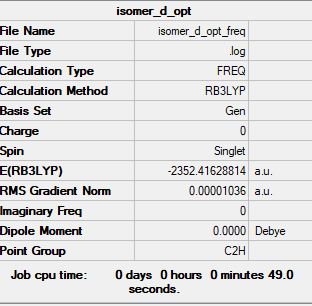
Isomer D (with trans terminal Br and bridging Cl ions)
Frequency file:File:ISOMER D OPT FREQ.LOG
Low frequencies --- -4.0848 -2.1692 -0.0022 0.0014 0.0023 1.2919 Low frequencies --- 17.7564 48.9703 72.9549
Item table:
Item Value Threshold Converged?
Maximum Force 0.000022 0.000450 YES
RMS Force 0.000010 0.000300 YES
Maximum Displacement 0.000778 0.001800 YES
RMS Displacement 0.000291 0.001200 YES
Predicted change in Energy=-1.891415D-08
optimised isomer d molecule |
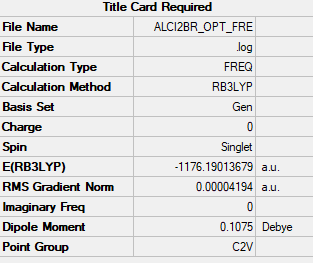
AlCl2Br monomer
Frequency file:File:ALCI2BR OPT FRE.LOG
Low frequencies --- -0.0029 -0.0023 0.0029 1.3569 3.6367 4.2604 Low frequencies --- 120.5042 133.9178 185.8950
Item table:
Item Value Threshold Converged?
Maximum Force 0.000081 0.000450 YES
RMS Force 0.000042 0.000300 YES
Maximum Displacement 0.001588 0.001800 YES
RMS Displacement 0.000974 0.001200 YES
Predicted change in Energy=-1.810813D-07
optimised isomer d molecule |
Good structure information throughout and correct implementation of the pseudopotential! Your isomers and assigned symmetries are also correct. Smf115 (talk) 08:25, 22 May 2019 (BST)
The energy of the isomers
E(B)=-2352.40601 a.u =-6175065kJ/mol
E(D)=-2352.41627 a.u =-6175092kJ/mol
E(D-B)=-0.01026 a.u =-27 kJ/mol
The isomer D is more stable in energy as the bridging atoms are Cl, which have 3p orbitals interact with sp2 Al orbitals and gives a better overlap.
The dissociation energy for the isomer D into 2AlCl2Br
ΔE=[E(AlCl2Br)+E(AlCl2Br)]-E(isomer D)=0.03547 a.u= 93.1 kJ/mol
The isomer D is more stable than the isolated monomer as the dissociation energy is positive, which means it is endothermic to dissociate into 2AlCl2Br.
Both calculations are correct. You needed to consider the accuracy of your final value here though (nearest 1 kJmol-1 and I think at some point have had a rounding error. To improve, it would have been nice to see a suggestion as to why the dimer is more stable. Smf115 (talk) 08:30, 22 May 2019 (BST)
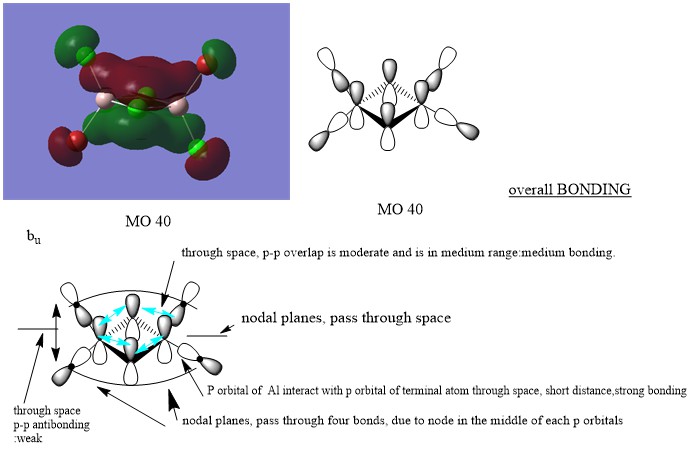
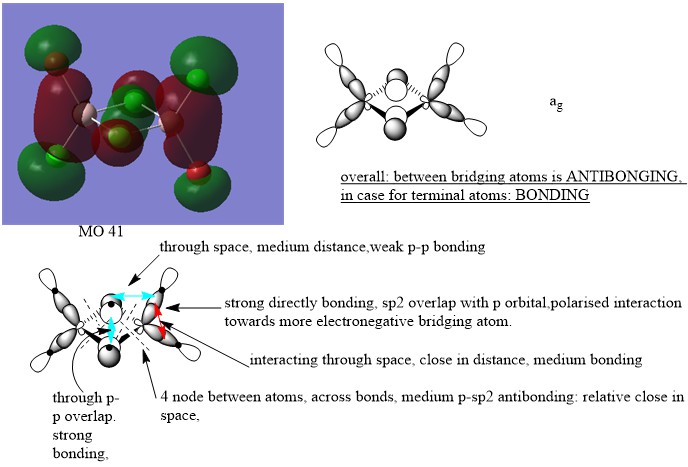
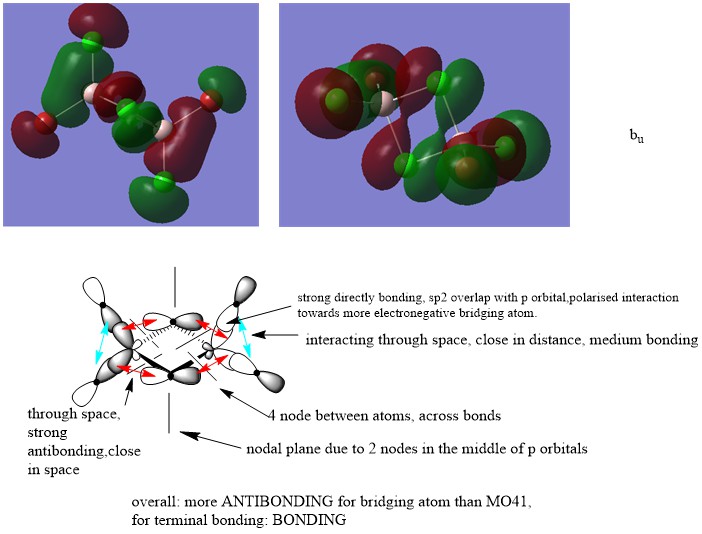
LCAO for selected MOs
MO 40
MO 41
MO 43
Nice attempt at constructing the LCAOs and analysing the interactions. You've highlighted the main interactions and have made a good argument when evaluating the MO character. There are mistakes though, particularly for MO 41 and 43 you should consider whether there is any contribution from the Al at all. To improve you could also present your annotations a bit clearer, the nodal planes aren't labelled very well and I think you have made mistakes when labelling them at places. Smf115 (talk) 08:50, 22 May 2019 (BST)
Overall, a nice report with good supporting calculations. Smf115 (talk) 08:50, 22 May 2019 (BST)