Rep:Mod:mx3118
NH3
test molecule |
File:XUMENGHAN NH3 OPTF POP.LOG
Summary
Calculation method
RB3LYP
Basis set
6-31G(d,p)
E(RB3LYP) in a.u.
-56.55777
RMS gradient norm in a.u.
0.00000485
Point group
C3v
Key structural and charge information
N-H bond length (in Ångstrom): 1.02 (accurate to ≈ 0.01 Ångstrom)
H-N-H bond angle (in Degree): 106 (accurate to ≈ 1 Degree)
Charge on N atom: -1.125
Charge on H atoms: 0.375
Note: The charges on N atom and H atom are shown as expected. The N atom should have a relatively more negative charge due to its higher electronegativity compared to H atom, H atom should have a relatively more positive charge.
Item table
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
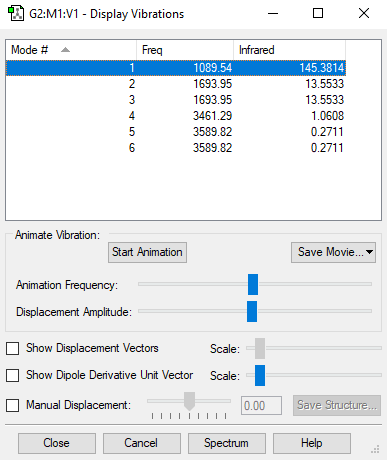
Screenshot of the display vibrations
Vibration data
1 2 3
A1 E E
Frequencies -- 1089.5366 1693.9474 1693.9474
IR Inten -- 145.3814 13.5533 13.5533
4 5 6
A1 E E
Frequencies -- 3461.2932 3589.8170 3589.8170
IR Inten -- 1.0608 0.2711 0.2711
Answers to the questions about vibrations and charges
1. From the 3N-6 rule, there are (3N-6=3*4-6=6) modes.
2. There are two sets of denegerate modes with wavenumbers (in cm-1): 1693.95 and 3589.82.
3. Bending modes have wavenumbers (in cm-1): 1089.54, 1693.95, 1693.95; stretching modes have wavenumbers (in cm-1): 3461.29, 3589.82, 3589.82.
4. The stretching mode with wavenumber (in cm-1) 3461.29 is highly symmetric.
5. The bending mode with wavenumber (in cm-1) 1089.54 is known as the "umbrella" mode.
6. Two bands are expected to show in an experimental spectrum of gaseous ammonia. Since the IR spectrum mainly records the stretching frequencies, and there are two non-degenerate values for stretching frequency.
N2
test molecule |
File:XUMENGHAN N2 OPTF POP.LOG
Summary
Calculation method
RB3LYP
Basis set
6-31G(d,p)
E(RB3LYP) in a.u.
-109.52413
RMS gradient norm in a.u.
0.00013245
Point group
Dinfh
Key structural and charge information
N-N bond length (in Ångstrom): 1.11 (accurate to ≈ 0.01 Ångstrom)
Charge on both N atoms: 0.000
Note: The charges on both N atoms are shown as expected. They both should have a neutral charge, since the electron density is shared between same atoms equally.
Item table
Item Value Threshold Converged?
Maximum Force 0.000229 0.000450 YES
RMS Force 0.000229 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000101 0.001200 YES
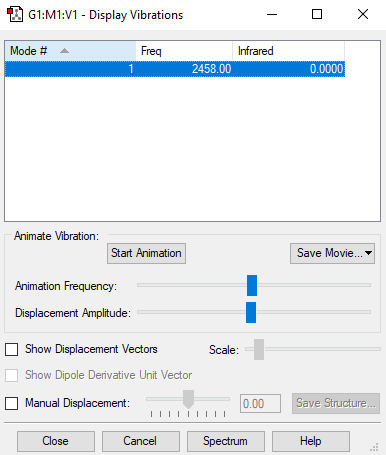
Screenshot of the display vibrations
Vibration data
1
SGG
Frequencies -- 2457.9991
IR Inten -- 0.0000
Mono-metallic TM complex that coordinates N2
Identifier and link for the structure
RANLUO
"https://www.ccdc.cam.ac.uk/structures/search?pid=csd:RANLUO"
N-N bond length
The optimised N-N bond length (in Ångstrom): 1.1054
The N-N bond length in crystal structure (in Ångstrom): 1.1122
The bond lengths are different. The N2 in the crystal is connected directly onto an electropositve Ni atom, electron density and hence repulsion around the two N atoms increase. This could potentially cause the lengthing of the N-N bond.
H2
test molecule |
File:XUMENGHAN H2 OPTF POP.LOG
Summary
Calculation method
RB3LYP
Basis set
6-31G(d,p)
E(RB3LYP) in a.u.
-1.17854
RMS gradient norm in a.u.
0.00040858
Point group
Dinfh
Key structural and charge information
H-H bond length (in Ångstrom): 0.74 (accurate to ≈ 0.01 Ångstrom)
Charge on both H atoms: 0.000
Note: The charges on both H atoms are shown as expected. They both should have a neutral charge, since the electron density is shared between same atoms equally.
Item table
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
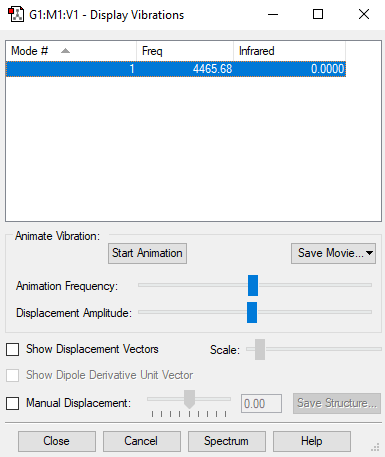
Screenshot of the display vibrations
Vibration data
1
SGG
Frequencies -- 4465.6824
IR Inten -- 0.0000
The Haber-Bosch process
Equation for this process
N2 + 3H2 -> 2NH3
Energy of reactants and product in a.u.
E(NH3)= -56.5577687
2*E(NH3)= 2*(-56.55776873) = -113.1155375
E(N2)= -109.5241287
E(H2)= -1.1785387
3*E(H2)= 3*(-1.17853870) = 3*( -1.17853870) = -3.5356161
Energy for this reaction
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -113.1155375 - (-109.52412866--3.5356161) = -0.0557927 (a.u.) = -0.0557927*2625.5 (kJ/mol) = -146.5(kJ/mol)
Note: hence from the energy of conversion, it is shown that this is a exothermic reaction, indicating that the ammonia product is more stable than the gasoues reactants.
My chosen molecule Cl2
test molecule |
File:XUMENGHAN CL2 OPTF POP.LOG
Summary
Calculation method
RB3LYP
Basis set
6-31G(d,p)
E(RB3LYP) in a.u.
-920.27125
RMS gradient norm in a.u.
0.17962
Point group
Dinfh
Key structural and charge information
Cl-Cl bond length (in Ångstrom): 2.04 (accurate to ≈ 0.01 Ångstrom)
Charge on both Cl atoms: 0.000
Note: The charges on both Cl atoms are shown as expected. They both should have a neutral charge, since the electron density is shared between same atoms equally.
Item table
Item Value Threshold Converged?
Maximum Force 0.000015 0.000450 YES
RMS Force 0.000015 0.000300 YES
Maximum Displacement 0.000043 0.001800 YES
RMS Displacement 0.000060 0.001200 YES
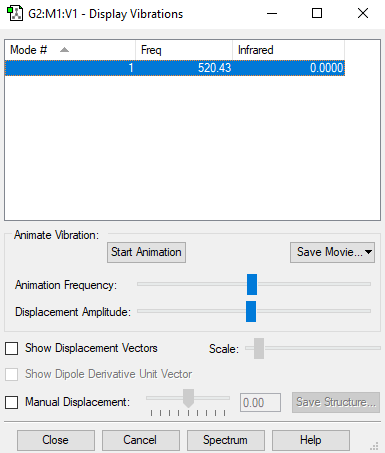
Screenshot of the display vibrations
Vibration data
1
SGG
Frequencies -- 520.4311
IR Inten -- 0.0000
Molecular orbitals in interest
First MO
This is a bonding MO filled with two electrons, since it is formed by in-phase combination of the 1s atomic orbitals of the two Cl atoms. This is also a sigma MO, since the two atomic orbitals overlap end-on. This MO has a comparatively very low energy at -101.60298, since its composite atomic orbitals are very close to the nuclues and hence feel a strong interaction and are buried deep down in energy. This MO is neither in the HOMO or the LUMO region. The electrons in this orbital will not be involved in bonding.
Second MO
This is a bonding MO filled with two electrons, since it is formed by in-phase combination of the 2p atomic orbitals of the two Cl atoms. This is a sigma MO, since the overlap of two AO is end-on. This MO has a higher energy, since the 2p orbitals are further away from the nucleus and at higher energy. This MO is neither in the HOMO or the LUMO region. The electrons in this orbital will not be involved in bonding.
Third MO
This is a bonding MO filled with two electrons, since it is formed by in-phase combination of the 2p atomic orbitals of the two Cl atoms. This is a pi MO, since the overlap of two AO is now parallel. This MO has a further increased energy, since the parallel overlap is less effective than the end-on overlap. This MO is neither in the HOMO or the LUMO region. The electrons in this orbital will not be involved in bonding.
Fourth MO
This is an pi* anti-bonding orbital filled with two electrons, since orbital combination of two 3p atomic orbitals is out-of-phase. This is the HOMO, hence the electrons in this MO have the highest energy. Hence this MO provide the most-readily-donated electrons in reaction.
Fifth MO
This is an sigma* anti-bonding orbital with no electrons, since orbital combination of two 3s atomic orbitals is out-of-phase. This is the LUMO. This is the ideal (lowest-energy) empty MO that can accept further electrons in reaction.
Independence -- study on CO2
test molecule |
File:XUMENGHAN O2 OPTF POP.LOG
Summary
Calculation method
RB3LYP
Basis set
6-31G(d,p)
E(RB3LYP) in a.u.
-150.25742
RMS gradient norm in a.u.
0.00001829
Point group
Dinfh
Key structural and charge information
O-O bond length (in Ångstrom): 1.22 (accurate to ≈ 0.01 Ångstrom)
Charge on both O atoms: 0.000
Note: The charges on both O atoms are shown as expected. They both should have a neutral charge, since the electron density is shared between same atoms equally.
Item table
Item Value Threshold Converged?
Maximum Force 0.000032 0.000450 YES
RMS Force 0.000032 0.000300 YES
Maximum Displacement 0.000019 0.001800 YES
RMS Displacement 0.000027 0.001200 YES
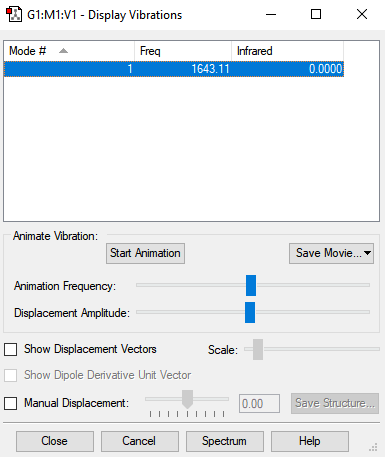
Screenshot of the display vibrations
Vibration data
1
SGG
Frequencies -- 1643.1143
Red. masses -- 15.9949
Frc consts -- 25.4430
IR Inten -- 0.0000
Marking
Note: All grades and comments are provisional and subjecct to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have recieved your grade from blackboard.
Wiki structure and presentation 0.5/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES - but all your jmols are labelled 'test molecule' which is not informative at all.
NH3 1/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES, however the important part is not that stretching or bending vibrations are generally favoured in IR. Looking at the intensities reveals that the stretching vibrations have very low ones and will not be present in the experimental spectrum.
N2 and H2 0.5/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES, you could have explained that the charges are 0 as the electronegativities are equal.
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 4/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
You have done a good job of presenting this information, well done! The first 3 MOs you showed are neither bonding nor anti-bonding. They are non-bonding because the Mos involved are not overlapping and interacting with each other. You explained this yourself and just gave them wrong names. You could have commented that the second and third MO are degenerate. The fish MO is not based on 3s orbitals but 3p orbitals.
Independence 1/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or
YES - the title of this sections is mismatching with the actual molecule you calculated!
Do some deeper analysis on your results so far