Rep:Mod:musiq-child
Module 1: Molecular Mechanics and Semi-Empirical MO Methods
All Geometries/Energies are derived using the ChemBio3D Suite/MM2 Force Field, unless otherwise specified. Also, the methods used below do not account for kinetic control type structures, i.e. transition states, as they are unable to process these states without full knowledge of their geometries/energies already to optimize further.
Molecular Mechanics: Basic Techniques
The Hydrogenation of Cyclopentadiene Dimer
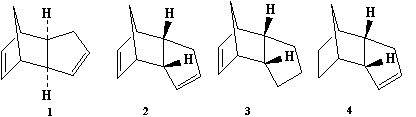
Cyclopentadiene readily dimerises at room temperature to give either dimer 1 (exo-form) or product 2 (endo-form), whereby the endo form is specifically produced. [1] Subsequent hydrogenation gives either initial dihydro derivative 3 or 4, followed by the tetrahydro derivative after prolonged hydrogenation.
First we will look at the optimised geometries of the exo and endo forms of the cyclopentadiene dimer:
 |
 |
| Total Energy/kcal mol-1 | |
|---|---|
| Exo dimer | 31.8831 |
| Endo dimer | 34.0134 |
As already stated, the endo form is specifically produced. However, looking at the total energies of both dimers, it is shown that the exo dimer is the more thermodynamically stable product, with a lower total energy; this is due to the lack of steric repulsion between each cyclopentadiene ring contributing to the whole dimer, compared with the endo dimer, which has the rings facing each other as you can see from the image above. Thus we can deduce that the cyclopentadiene dimerisation does not proceed with thermodynamic control, but with kinetic control instead.
Secondly, let's look at the optimised geometries of the initial dihydro derivatives 3 and 4:
 |
 |
| Dihydro Derivative 3 | Dihydro Derivative 4 | |
|---|---|---|
| Stretch | 1.2231 | 1.1034 |
| Bend | 18.8793 | 14.5151 |
| Stretch-Bend | -0.7560 | -0.5464 |
| Torsion | 12.2050 | 12.5123 |
| Non-1, 4 VDW | -1.5240 | -1.0470 |
| 1, 4 VDW | 5.7412 | 4.4985 |
| Dipole-Dipole | 0.1631 | 0.1408 |
| Total Energy | 35.9317 | 31.1767 |
The total energies calculated above allow us to make a predicition based on thermodynamic considerations/control of which dihydro derivative 3 or 4 will be preferred following intial hydrogenation. As the total energy of derivative 3 is greater than that of 4, 4 is the less strained and therefore the preferred isomer; this leads to the idea that the double bond adjacent to the CH2 bridge is easier to hydrogenate. Comparing the relative contributions to the total energy, most are fairly similar; some fair differences in the 1, 4 VDW (Van der Waals) and torsion contributions. However the main difference is in the relative contribution of bend energy. The difference is 4.3642 kcal mol-1, a fairly large amount given the sizes of the contributions, and therefore the major factor in the relative stability of derivative 4 over 3. The stretch contributions are relatively minimal in difference, and there is no H-bonded energy term.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
(Using MMFF94 Force Field for this section)
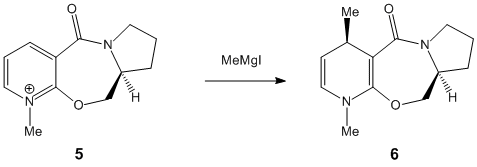 |
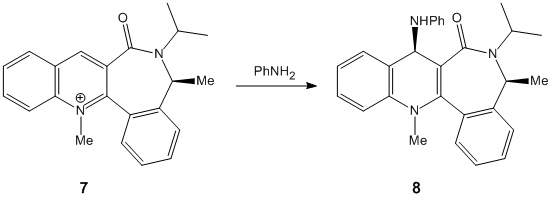 |
In both cases of the reactions above, there is a stereocontrolled nucleophilic addition to the pyridinium ring at the 4-position, generating products with the above absolute stereochemistry. Initially, this absolute stereochemistry at this position looks like it is strongly influenced by the neighbouring carbonyl group, with the incoming nucleophile approaching the pyridinium ring at the face opposite to the where the carbonyl group is located. In particular for 7 -> 8, it is likely that the phenyl ring adjacent to the pyridinium ring is also on the face opposite to nucleophilic attack, facilitating the attack and preventing steric hindrance to attack (if the attack is opposite to the carbonyl face/location). This suggests an endo-type isomeric form of the pyridinium reactant 7. This hypothesis can be investigated further by looking at the optimised geometries of the two pyridinium reactants 5 and 7:
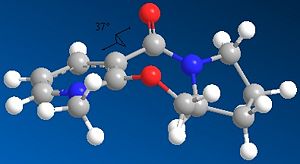
|
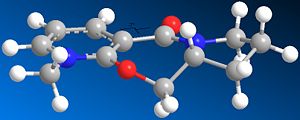
|
|---|
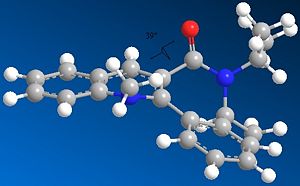 |
 |
The optimization was tried for reactant 5 with the MeMgI component, but the MMFF94 force field failed to recognise the Mg atom, as it didnt contain the necessary parameters to do so. When optimizing both reactants, it was found that there weren't many conformational changes in the 7- and 5-membered rings, with only slight total energy differences in changing a particular atom location/bond angle. The 7-membered ring was particularly inflexible in this case, and gave rise to only two energy minima for both reactants, one with the carbonyl pointing "upwards"/out of the page (stereospecifically), and the other more planar, with respect to the pyridinium ring, for reactant 5, and pointing "downwards"/into the page, for reactant 7.
| Reactant 5 | Reactant 7 | ||
|---|---|---|---|
| Correct C=O orientation conformer | 59.2193 | 113.136 | Incorrect C=O orientation |
| Lowest energy conformer | 57.4143 | 98.5257 | Lowest energy conformer with correct C=O orientation |
The energies however are not that much more different in terms of stability, and in fact as stated the higher energy minimum is the correct or "preferred" conformer for reaction 5 -> 6 as its carbonyl is pointing "upwards" and therefore has the correct geometry; however for 7 -> 8, the lower energy conformer has the correct carbonyl geometry. This is because in 5 -> 6, chelation-type control results from the -(+)MgI component coordinating to the carbonyl oxygen, and thus delivering the methyl group to the pyridinium ring with this preferred sterochemistry, which is the same as the carbonyl's. The carbonyl oxygen in this case is anti to the proton at the chiral centre, as indicated in 5/6. In 7 -> 8, diasterofacial selectivity controls the reaction; this involves the steric repulsion of the carbonyl moiety (and the O lone pairs), so that aniline reacts on the opposite face to this. Therefore the lowest energy conformer with its carbonyl pointing "downwards" slightly (dihedral angle with respect to the ring = -37o) promotes formation of 8. [2] [3]
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
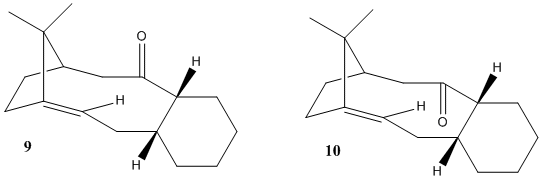
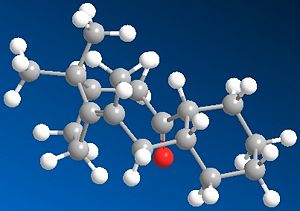
The two isomeric forms of the key intermediate in the Taxol synthesis (9 and 10) are atropisomers; this means that they're stereoisomers as a result of hindered rotation about single bonds, whereby the single bond in question is the C-C bond adjacent to the carbonyl function, but that further away from the cyclohexyl group. The result of this is the C=O can point either upwards or downwards. The two isomers were optimised:

|

|
|---|
|

|
|---|
| Isomer 9 | Isomer 10 | |
|---|---|---|
| Twist-Boat | 54.4002 | 54.3092 |
| Chair | 48.8999 | 49.5606 |
In both isomers, the chair form is the more stable conformer, and the energies are fairly indifferent in energy, proposing that the energy barrier to interconversion would be low and hence neither isomer is more stable than the other. However, it was discovered that for isomer 9, the cyclo-transannular-enone-type ring strucutre is sufficiently rigified so that the adjacent fused cyclohexyl ring is locked into the higher energy twist-boat conformation. For isomer 10, the opposite is true; the chair conformation is preferred. [4] Hence Paquette initially synthesised the higher energy isomer 9 with twist-boat conformation, which then isomerised on standing to the more stable isomer 10 with chair conformation. It should be noted that both isomers are relatively strained ring structures. However, the strain of these bridgehead olefins is actually less than that of the parent hydrocarbon; these alkenes are hyperstable! This is based on Olefin Strain (OS) energy measurements, which compare the strain of the olefin and its parent hydrocarbon, and is usually positive for e.g. molecules that follow Bredt's Rule. The OS values are negative for these olefins, and so they show high unreactivity as they are more stable being at these bridgehead positions (i.e. anti-Bredt molecules); this can be tied into the cage-type structure of these olefins. [5] Using the MMFF94 Force Field produces higher absolute energies of the total structures, therefore perhaps not accounting the OS energy and not as good a force field to use to optimize/minimize the geometries/energies.
Semi-Empirical Molecular Orbital Theory: Basic Techniques
Regioselective Addition of Dichlorocarbene
Part 1:
(Using MOPAC/PM6 alongside MM2 for this section)
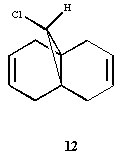
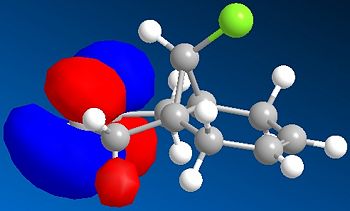
Compound 12 contains nucleophilic alkene groups that are susceptible to electrophilic addition by dichlorocarbene, or peracids. However, one alkene may be more nucleophilic than the other; this is investigated further using the MO's derived (specifically those below):
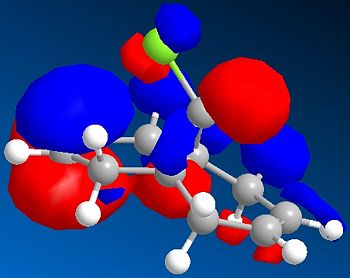 |
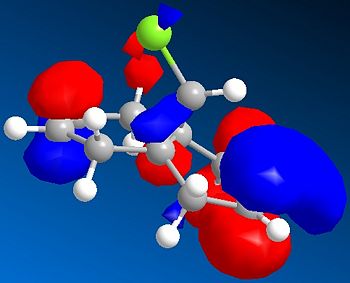 |
 |
 |
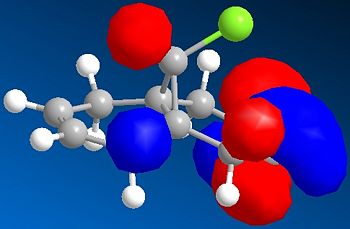 |
The HOMO of 12 shows us that the π-face of the alkene endo to the Cl atom has a significantly higher electron density compared to the exo-alkene, thus suggesting that the regioselective attack towards the electrophile dichlorocarbene occurs at this alkene. This can be explained by an antiperiplanar interaction between the Cl-C σ* orbital (LUMO+1) and the occupied exo-π orbital (HOMO-1), stabilising this π-MO and making the endo one more nucleophilic. The HOMO-1 π-orbital also is more diffuse and hence less reactive than that of the HOMO. Proof of this π-σ* negative hyperconjugation interaction is shown in the distances between either exo/endo double bond carbon and the central bridgehead carbon:
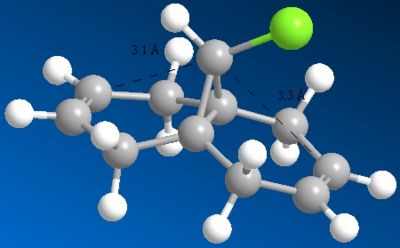
This interaction shortens the distance to the exo carbon by about 0.2Å compared to the endo carbon, in fairly good accordance with the literature value [6] and indicates a geometrical distortion of the bicyclic structure.
Part 2:
(Using MOPAC/PM6 and DFT-type Gaussian B3LYP/6-31 G(d,p) geometry optimization and frequency calculation alongside MM2 for this section)
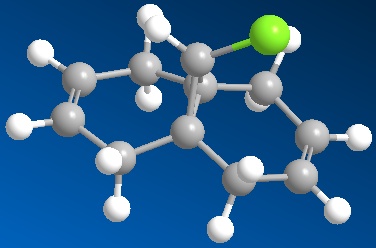 |
 |
The Cl-C bond can have a significant influence on the vibrational frequencies of molecules 12 and 13, albeit with some expected difference in effect. This was investigated using a DFT (Density Functional Theory) Gaussian approach, submitted to the SCAN computing cluster, and inspected using Gaussview 5.0.8c:
| Compound 12 | Compound 13 | |
|---|---|---|
| Cl-C stretch | 548.77 | 536.41 |
| endo C=C stretch | 1757.44 | 1758.05 |
| exo C=C stretch | 1737.02 | N/A |
The stretches for the diene and the monohydrogentaed derivative show a fairly weak (intensity) stretch at low wavenumber frequency, corresponding to the single-bonded Cl-C, and there is a fair difference in this value for 12 and 13. Both also show a very weak stretch at much higher wavenumber frequency, which corresponds to the C=C stretches with a much greater electron density in this type of bond compared to the Cl-C bond. It should be noted that the difference between both endo stretches is insignificant, but between endo and exo is significant, most likely reflecting stereoelectronic interactions in the compounds 12 and 13, i.e. the negative hyperconjugation above that decreases the electron density in the exo double bond.
References
- ↑ K. Alder and G. Stein, Liebigs Ann. Chem., 1933, 504, 210-257.DOI:10.1002/jlac.19335040115
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry., 1986, 51, 838.DOI:10.1021/jo.00356a016
- ↑ S. Leleu, C.; Papamicael, F. Marsais, G. Dupas, V.; Levacher, Vincent, Tetrahedron: Asymmetry, 2004, 15, 3919-3928.DOI:10.1016/j.tetasy.2004.11.004
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 32, 319-322.DOI:10.1016/j.tetasy.2004.11.004
- ↑ W. F. Maier and P. V. R. Schleyer, J. Am. Chem. Soc., 1981, 103, 1891-1900.DOI:10.1021/ja00398a003
- ↑ B. Halton, R. Boese and H. S. Rzepa, J. Chem. Soc., 1992, Perkin Trans. 2, 447-448.DOI:10.1039/P29920000447
