Rep:Mod:mpg2711
NH3 Molecule
Optimisation Data
Molecule: NH3
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy: -56.55776873 a.u.
RMS Gradient: 0.00000485 a.u.
Point Group: C3V
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Structural Data
N-H bond distance = 1.01798Å
H-N-H bond angle = 105.741°
NH3 Molecule |
Vibrational Data
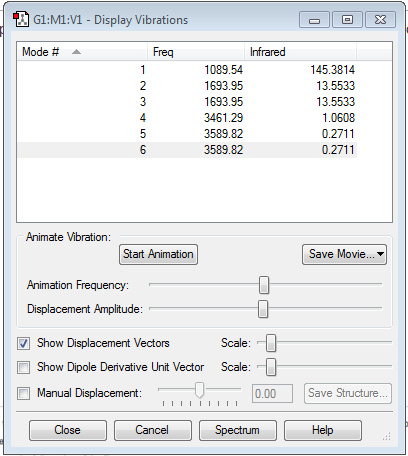
We would expect 6 vibrational modes from the 3N-6 rule, as there are 4 atoms in the molecule.
Modes 2 and 3 are degenerate in energy, as are modes 5 and 6.
Modes 1,2 and 3 are bending modes, and modes 4,5 and 6 are stretching modes.
Modes 1 and 4 are highly symmetric.
Mode 1 is the umbrella mode.
You would expect 2 bands in the experimental spectrum of ammonia. This is because only modes 1, 2 and 3 have energies high enough to be clearly visible in the spectrum. Since modes 2 and 3 are degenerate, they will give one peak.
Charge Data
The hydrogen atoms have a charge of 0.375
The nitrogen atom has a charge of -1.125
I would expect the hydrogen to have a positive charge, and the nitrogen to have a negative charge, as nitrogen is a more electronegative element than hydrogen, and so it will draw the electron density towards itself.
N2 Molecule
Optimisation Data
Molecule: N2
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy: -109.52412868 a.u.
RMS Gradient: 0.00000060 a.u.
Point Group: D*H
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Structural Data
N-N bond length: 1.10550Å
N2 Molecule |
Vibrational Data
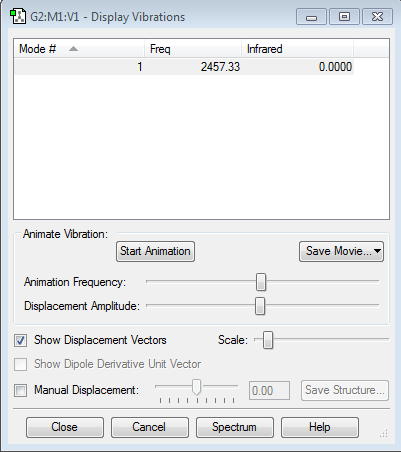
There is only one high frequency vibration, from the 3N-5 rule for linear molecules. It has no value in the infrared specturm, as there is no change of dipole moment.
Charge Data
The N2 molecule has no overall charge distribution, as both of the nitrogen atoms are identical.
H2 Molecule
Optimisation Data
Molecule: H2
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy: -1.17853936 a.u.
RMS Gradient: 0.00000017 a.u.
Point Group: D*H
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Structural Data
H-H bond length: 0.74279Å
H2 Molecule |
Vibrational Data
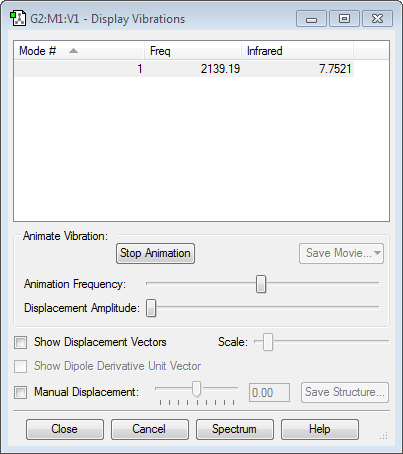
There is only one high frequency vibration, from the 3N-5 rule for linear molecules. It has no value in the infrared specturm, as there is no change of dipole moment.
Charge Data
The H2 molecule has no overall charge distribution, as both of the hydrogen atoms are identical.
Molecular Calculations
E(NH3)= -56.55776873 a.u.
2*E(NH3)= -113.11553746 a.u.
E(N2)= -109.52412868 a.u.
E(H2)= -1.17853936 a.u.
3*E(H2)= -3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907 a.u. = -146.47848285 kJ/mol
This is the energy for converting hydrogen and nitrogen gas into ammonia gas. It shows that the ammonia product is more stable than the reactants.
This differs from data found in the NIST Online Database. Using the information given there, the energy change during the formation of two moles of ammonia is -91.8 kJ/mol.[1]
This difference is caused by the fact the experiments from which the database value is calculated have factors such as catalysts present, which are not accounted for in the simulation.
Project Molecule: CN-
Optimisation Data
Molecule: CN-
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy: -92.82453153 a.u.
RMS Gradient: 0.00000704 a.u.
Point Group: C*V
Item Value Threshold Converged? Maximum Force 0.000012 0.000450 YES RMS Force 0.000012 0.000300 YES Maximum Displacement 0.000005 0.001800 YES RMS Displacement 0.000008 0.001200 YES
Structural Data
C-N bond length: 1.18409Å
CN- Molecule |
Vibrational Data
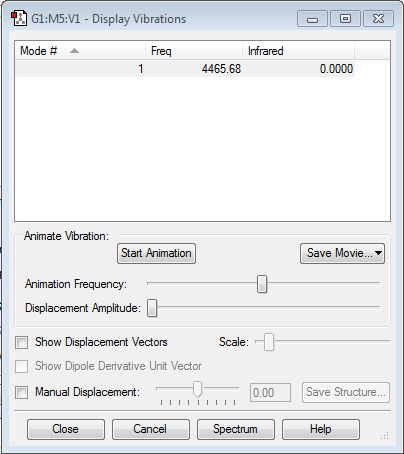
The molecule displays a single vibration mode, following the 3N-5 rule for linear molecules. It is active in the infrared spectrum as there is a change in the dipole moment.
Charge Data
The carbon atom has a charge of -0.246.
The nitrogen atom has a charge of -0.754.
This result is expected, as nitrogen is a more electronegative element than carbon, and so it will draw the electron density towards itself.
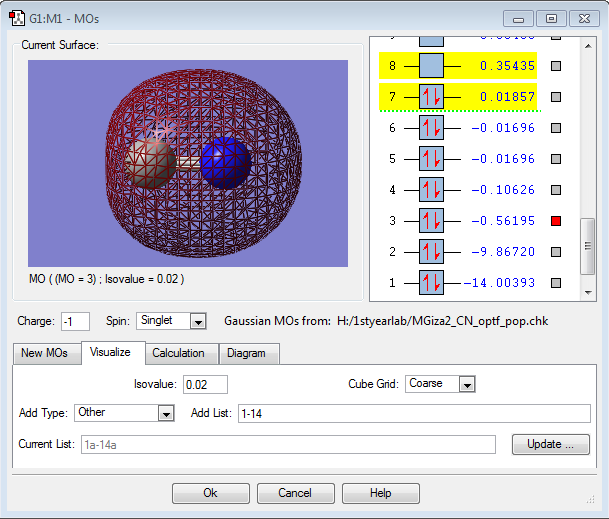
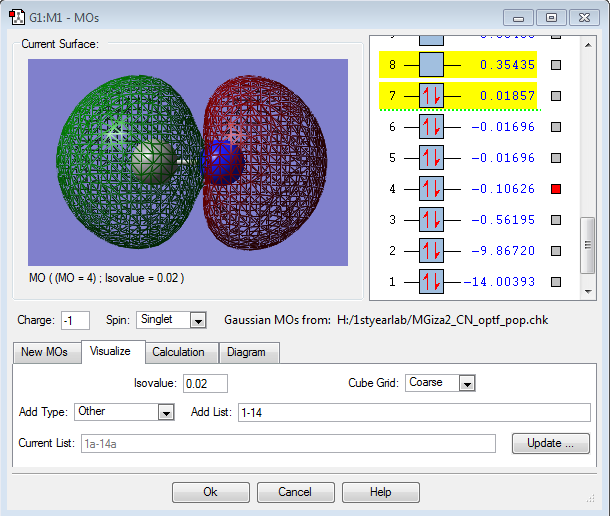
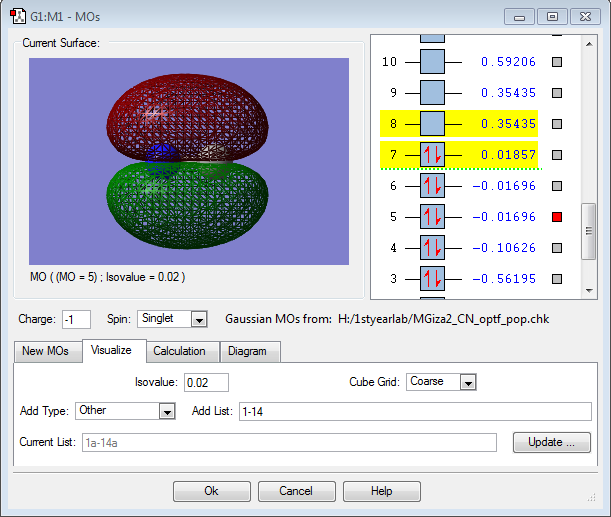
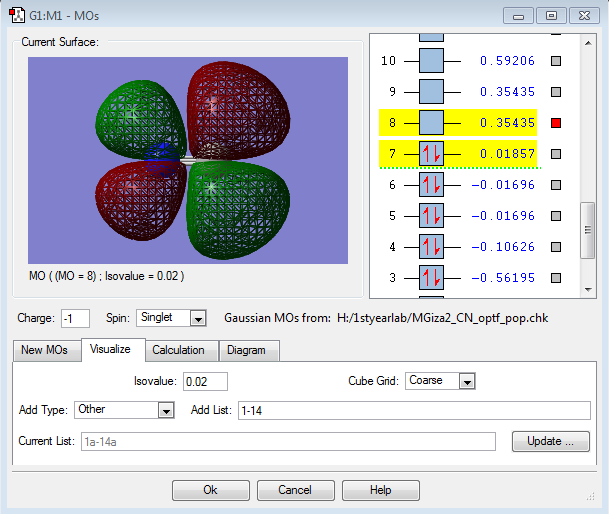
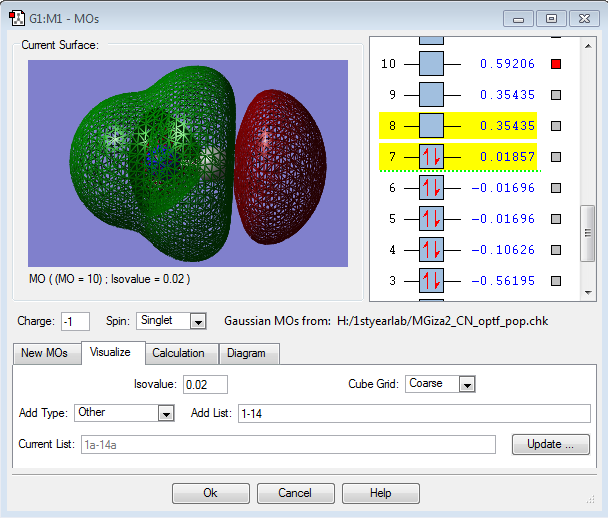
MO Data
This is the 3σ MO, formed from the in-phase overlap of the 2s orbitals of the two atoms. It is a filled bonding orbital, and shows that there is more electron density around the nitrogen atom in the bonding situation. It is relatively deep in energy.
This is the 4σ* MO, formed from the out-of-phase overlap of the 2s orbitals of the two atoms. It is a filled antibonding orbital, and shows there is more electron density around the carbon atom in the antibonding situation.
This is the 1π MO, formed from the in-phase overlap of two p orbitals. It is a filled bonding orbital, actively contributing to form the triple bond between the carbon and nitrogen. It is close to the HOMO/LUMO region.
This is the 2π* MO, formed from the out-of-phase overlap of two p orbitals. It is an empty antibonding orbital, and it is also the LUMO for the molecule. It shows that there is more electron density around the carbon atom in the antibonding situation.
This is the 6σ* MO, formed from the out-of-phase overlap of two p orbitals. It is an empty antibonding orbital, and shows an interesting, layered electron distribution, with a large lobe near the carbon atom.
References
1 : http://webbook.nist.gov/cgi/cbook.cgi?Name=ammonia&Units=SI&cTG=on&cTC=on&cTR=on
- ↑ 1