Rep:Mod:mod3 christopherkaye
Christopher Kaye, ck1510, Module 3 Computational
Extension granted by Dr Hunt to 16th March.
Introduction
This module uses Gaussian software to model transition states of reactions. A transition state is the maximum saddle point on a potential energy surface. These states are not observable spectroscopically as they are so short-lived, assumed to be on a similar order of magnitude to bond vibrations - approximately 10-18 s. [1]
Investigating transitions states is helpful in understanding kinetics and mechanisms of reactions. Gaussian modelling allows geometries and energies of transition states to be predicted.
In this module, two reactions' transition states are being investigated: the Cope Rearrangement, and Diels-Alder.
Cope Rearrangement
The Cope rearrangement is a pericyclic reaction, specifically a [3+3] sigmatropic rearrangement. Like all pericyclic reactions, it is concerted in nature and passes through a transition state and no intermediates. In the case of 1,5-hexadiene, the transition state goes through a chair-like or a boat-like transition state [2] Gaussian was used to identify low energy conformers and then to attempt to find boat and chair transition states.
Optimisation of conformers
Using Hartree-Fock method and 3-21G basis set in each case, various conformers were chosen and optimised. The gauche3 conformer was found to be the lowest energy conformer due to the 'gauche effect' of London forces and favourable orbital interactions. Where sterics become more important, it is expected that antiperiplanar would become the most stable conformer. [3]
Optimisation of antiperiplanar1 1,5-hexadiene
DOI:10042/24051 Link to D-space optimisation output

 Using 'Symmetrize'(sic) the molecule was found to have C2 symmetry.
Using 'Symmetrize'(sic) the molecule was found to have C2 symmetry.
Optimisation of gauche4 1,5-hexadiene
By changing the conformation manually to a gauche linkage, and optimising as above, the following results summary was produced.
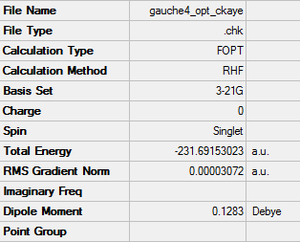
Link to .log file File:GAUCHE4 OPT CKAYE.LOG
Optimisation of gauche6 1,5-hexadiene
Link to .log file File:OPT GAUCHE6 CKAYE.LOG
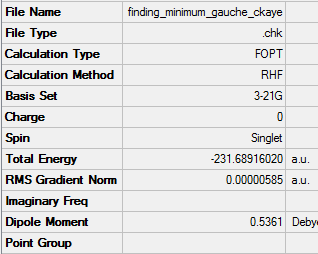
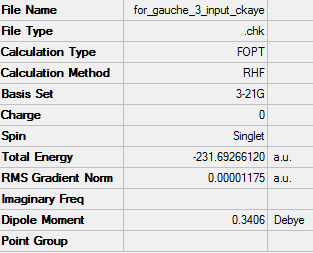
Optimisation of gauche3 1,5-hexadiene
Link to .log fileFile:FOR GAUCHE 3 INPUT CKAYE.LOG
Optimisation of gauche1 1,5-hexadiene
Link to .log file File:GAUCHE 1 CKAYE.LOG
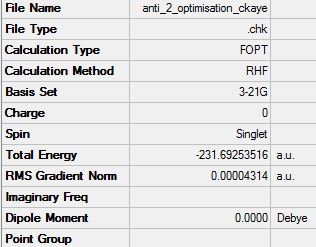
Optimisation of anti2 1,5-hexadiene
Link to .log file File:ANTI 2 OPTIMISATION CKAYE.LOG
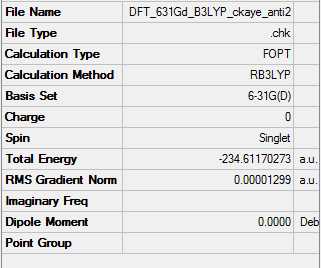
Higher optimisation of anti2 1,5-hexadiene
Link to .log file File:ANTI 2 HIGHER OPTIMISATION CKAYE.LOG
'Comparison' of two optimisations of anti2 1,5-hexadiene
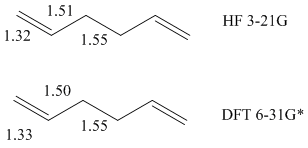
It is a major faux pas to directly compare optimisations using different basis sets. Both optimisations were carried out using Hartree-Fock method. The higher basis set yielded a much lower final energy, by approximately 3 atomic units. The bond lengths however are nearly identical, as can be seen below.
Frequency analysis of anti2
From the frequency analysis of the higher optimised structure of anti2 1,5-hexadiene, the output file confirmed that there were no negative frequencies, which ensures that the molecule is at an energy minimum.
Low frequencies --- -18.8360 -11.7255 -0.0010 -0.0004 0.0004 1.7210 Low frequencies --- 72.7084 80.1375 120.0085
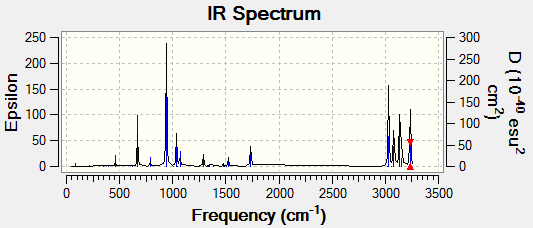
Predicted infrared spectrum of anti2
In the output file it can be seen that there are many vibrational modes. However, many have intensities of 0 because they do not involve a change in dipole moment, which is required for an interaction with electromagnetic field. Hence they do not appear on an IR spectrum.
Optimisation of chair transition state structure
An allylic H2CCHCH2 fragment was optimised using HF/3-21G basis set. This was later used to form the chair transition state below.
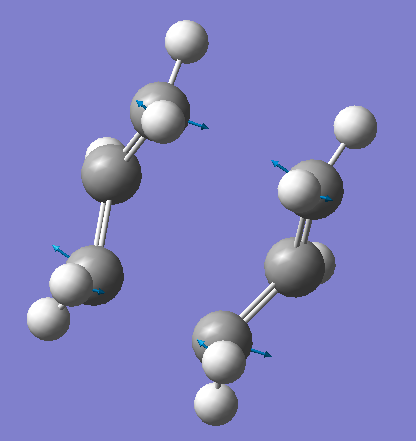
By fixing the bond forming/breaking distances of the terminal carbon atoms to approximately 2.2 Å and performing an opt+freq using the same basis set as before, but with TS(berny) selected and opt=noeigen, to allow imaginary frequencies. An imaginary frequency was observed at 818 cm-1, relating to the transition state. The displacement vectors of the vibration can be seen below
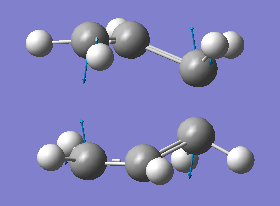
Intrinsic Reaction Co-ordinate (IRC) analysis of chair transition state from frozen co-ordinate

With 'forward only' steps. As the gradient approaches zero, the energy is being minimised as gaussian translates the molecule to minimise the energy.
Optimisation of boat transition state structure
By using the same allylic H2CCHCH2 fragment that was optimised using HF/3-21G basis set for the chair TS.
Using the same method as for the chair transition state.
Yielded an imaginary frequency at 840 cm-1 corresponding to the transition state
Activation energies
The data obtained from the transition states and the optimised anti2 molecule can be used to calculate the activation energy for each of the chair and the boat structures. The results are in kj mol-1 converted from Hartree atomic units
Diels-Alder
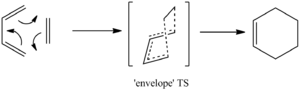
Introduction
A Diels-Alder reaction is a [4+2] cycloaddition between a conjugated diene and a dienophile, forming a 6-membered ring. The driving force of the reaction is likely to be enthalpic in nature due to the formation of (stronger) sigma bonds at the 'expense' of (weaker) pi bonds. The enthalpic contribution usually overrides any entropic disfavourability[4] (from the inherent ordering of two molecules into one).
Here, the diene is butadiene and the dienophile is ethylene.
Molecular orbital analysis is important because the symmetry of the reactant MOs is vital to establishing if the reaction occurs or not. If the reactants differ in symmetry then the required orbital overlap does not occur and so the reaction does not. (Woodward Hoffman rules)
Optimisation
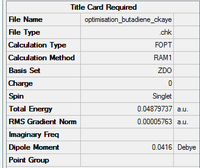
Using semi-empirical AM1 basis set, a molecule of cis-butadiene was optimised.
File:OPTIMISATION BUTADIENE CKAYE.LOG Link to .log file of optimisation of butadiene
MO of cis-butadiene
The molecular orbitals were obtained, with HOMO and LUMO of particular interest.
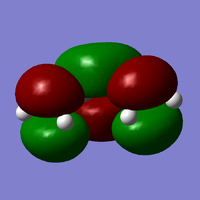

As can be seen above, the LUMO is symmetric with respect to a plane down the middle of the molecule. The HOMO however is antisymmetric since it does not have a plane of symmetry.
The relative energies of the MOs of cis-butadiene can be seen below.
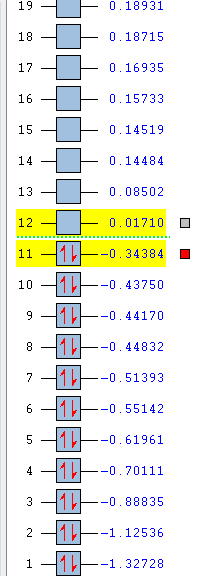
Formation of TS
A guess structure of the TS was obtained using 2.20 Å fixed distance.
File:TS OPT CKAYE.LOG Link to .log file of TS optimisation

An imaginary frequency was found at 956 cm-1
Low frequencies --- -956.2090 -0.0435 -0.0282 -0.0032 1.6785 3.3690 Low frequencies --- 4.2132 147.3980 246.6385
Terminal carbon-carbon distances = 2.12 Å
Regioselectivity of Diels Alder reaction
Using maleic anhydride to explore endo vs exo TS
Endo
File:OPTIMISATION CYCLOHEXAMAL ENDO CKAYE 2.LOG Link to .log file
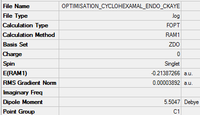

Exo
File:OPTIMISATION CYCLOHEXAMAL EXO TS CKAYE.LOG Link to .log file

Conclusion
Had all the calculations been done in time, transition state bond lengths could have been compared to know bond lengths to indicate the extent of bond formation / bonding interactions.
Reactions of the pi and pi* orbitals of ethylene and the HOMO of cis-butadiene. The formation of C-C sigma bonds create an asymmetric MO. The HOMO of the TS is found to be asymmetric, allowing the reaction to occur.
References
- ↑ Biochemistry by Reginald H. Garrett, Charles M. Grisham
- ↑ Futher Perspectives in Organic Chemistry by CIBA Foundation Symposium
- ↑ Carbohydrate Chemistry and Biochemistry: Structure and Mechanism by M. L. Sinnott
- ↑ Biotechnology: Bridging Research and Applications edited by Daphne Kamely, Ananda M. Chakrabarty, Steven E. Kornguth