Rep:Mod:mmo116 2
EX3 Section
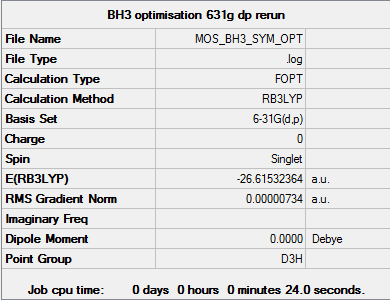
BH3
B3LYP/6-31G(d,p)
Summary table
"Item" table from optimisation
Item Value Threshold Converged? Maximum Force 0.000015 0.000450 YES RMS Force 0.000010 0.000300 YES Maximum Displacement 0.000058 0.001800 YES RMS Displacement 0.000038 0.001200 YES
Frequency analysis log file MOS_bh3_freq.log
Low frequencies
Low frequencies --- -10.3498 -3.4492 -1.2454 -0.0055 0.4779 3.2165 Low frequencies --- 1162.9519 1213.1527 1213.1554
Jmol image
optimised BH3 molecule |
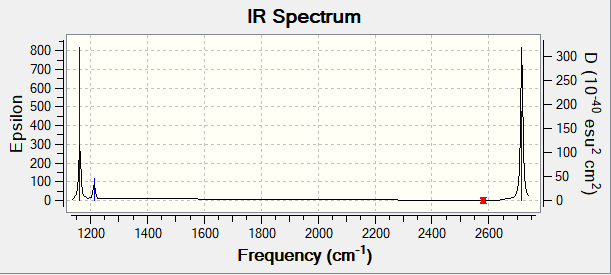
Vibrational Spectrum for BH3
| Frequency / cm-1 | Intensity | Symmetry | IR Active? | Type of Vibration |
|---|---|---|---|---|
| 1163 | 93 | A2" | Yes | Bend - out-of-plane |
| 1213 | 14 | E' | Slightly | Bend |
| 1213 | 14 | E' | Slightly | Bend |
| 2582 | 0 | A1' | No | Stretch - Symmetrical |
| 2716 | 126 | E' | Yes | Stretch - Asymmetrical |
| 2716 | 126 | E' | Yes | Stretch - Asymmetrical |
IR spectrum of BH3
There are only 3 peaks in the IR spectrum, despite there being 6 vibrational modes. In order to be IR active, there must be a change in dipole caused by the vibrations. The vibration at a frequency of 2582 cm-1 is symmetrical stretching and doesn't cause a change of dipole, so won't be seen in the spectrum. In addition to this, there are two sets of doubly degenerate vibrational modes, meaning you see two less peaks than the number of IR active modes. This adds up to seeing three peaks for all the six vibrational modes of the molecule.
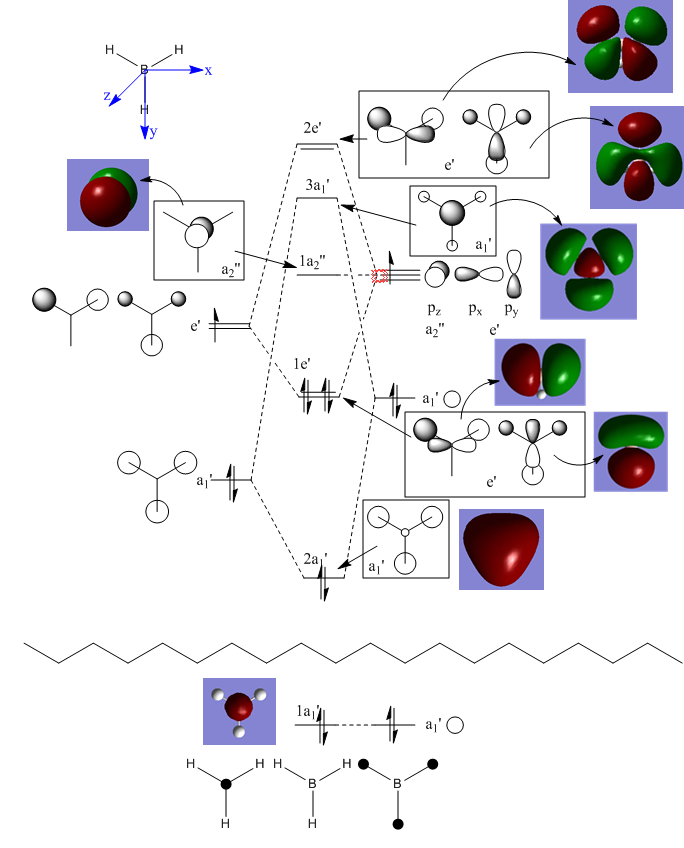
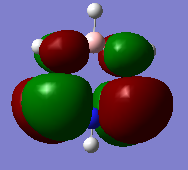
MO diagram for BH3
Are there any significant differences between the real and LCAO MOs?
There aren't any significant differences between the real and LCAO MOs - all nodes are in the same places and there are the same number of modes. Also, the shapes are similar - with the real MOs looking like the LCAOs with the orbitals overlapping, as expected. However, the more complicated the MO, the less like the LCAO the real MOs look.
What does this say about the accuracy and usefulness of qualitative MO theory?
This means that qualitative MO theory is useful - it gives a good indication of where orbitals may lie and what they should look like. However, the accuracy depends on how much the person qualitatively building the MO diagram understands how much splitting will occur and how much each fragment orbital contributes to the molecular orbitals in terms of shape. It also depends on how complicated the MO is.
Ng611 (talk) 18:02, 17 May 2018 (BST) From comparing the calculated and qualitative MOs, are there any differences at all between them at all?
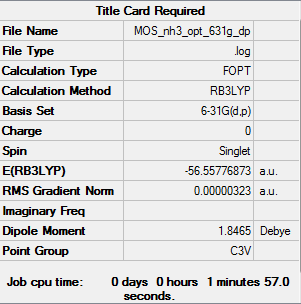
NH3
B3LYP/6-31G(d,p)
Summary table
"Item" table from optimisation
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES
Frequency analysis log file MOS_nh3_freq.log
Low frequencies
Low frequencies --- -8.5646 -8.5588 -0.0041 0.0455 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
Jmol image
optimised NH3 molecule |
NH3BH3
B3LYP/6-31G(d,p)
Summary table
"Item" table from optimisation
Item Value Threshold Converged? Maximum Force 0.000123 0.000450 YES RMS Force 0.000058 0.000300 YES Maximum Displacement 0.000515 0.001800 YES RMS Displacement 0.000296 0.001200 YES
Frequency analysis log file MOS_nh3bh3_freq.log
Low frequencies
Low frequencies --- 0.0004 0.0010 0.0011 18.3311 28.2136 39.8469 Low frequencies --- 266.4225 632.3934 639.8186
Jmol image
optimised NH3BH3 molecule |
E(NH3)= -56.55777 a.u.
E(BH3)= -26.61532 a.u.
E(NH3BH3)= -83.22469 a.u.
ΔE=E(NH3)BH3))-[E(NH3))+E(BH3))]
ΔE=(-83.22469)-((-56.55777)+(-26.61532))
Association Energy (a.u.) = -0.05160 a.u.
Association Energy (kJ/mol) = -135 kJ/mol
The B-N dative bond is a relatively weak bond.
Association bond energies are the same magnitude, but opposite sign to dissociation bond energies. Strong bonds, such as C-F and CO (triple bond) have a dissociation energy of 536 kJ/mol and 1075 kJ/mol respectively. The C-C bond in ethane is 368 kJ/mol. Compared to these, the -135 kJ/mol dissociation energy of B-N bond shows the bond is weak. [1]
Ng611 (talk) 18:01, 17 May 2018 (BST) Good calculation and well done for providing a citation for your comparison value. In future, consider using a textbook, databook or a paper for your bond enthalpy as they're considered more 'reliable'.
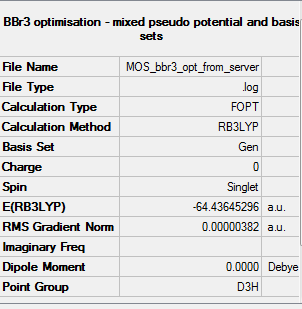
BBr3
B3LYP/6-31G(d,p)LANL2DZ
Summary table
"Item" table from optimisation
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000023 0.001200 YES
Frequency analysis log file File:MOS_BBR3_FREQ_new_from_server.log
Low frequencies
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
Jmol image
optimised BBr3 molecule |
Project section: Investigating Aromaticity
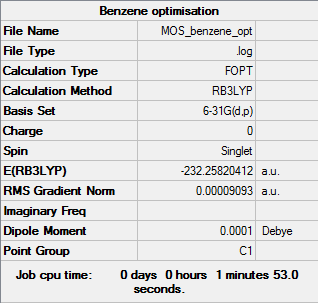
Benzene
B3LYP/6-31G(d,p)
Summary table
"Item" table from optimisation
Item Value Threshold Converged? Maximum Force 0.000198 0.000450 YES RMS Force 0.000082 0.000300 YES Maximum Displacement 0.000849 0.001800 YES RMS Displacement 0.000305 0.001200 YES
After setting the symmetry to D6h:
New Summary table
New "Item" table from optimisation
Item Value Threshold Converged? Maximum Force 0.000194 0.000450 YES RMS Force 0.000077 0.000300 YES Maximum Displacement 0.000824 0.001800 YES RMS Displacement 0.000289 0.001200 YES
Frequency analysis log file MOS_benzene_freq.log
Low frequencies
Low frequencies --- -2.1456 -2.1456 -0.0089 -0.0042 -0.0041 10.4835 Low frequencies --- 413.9768 413.9768 621.1390
Jmol image
optimised benzene molecule |
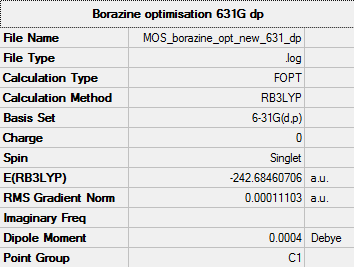
Borazine
B3LYP/6-31G(d,p)
Summary table
"Item" table from optimisation
Item Value Threshold Converged? Maximum Force 0.000130 0.000450 YES RMS Force 0.000051 0.000300 YES Maximum Displacement 0.000597 0.001800 YES RMS Displacement 0.000239 0.001200 YES
After setting the symmetry to D3h:
New Summary table
New "Item" table from optimisation
Item Value Threshold Converged? Maximum Force 0.000107 0.000450 YES RMS Force 0.000048 0.000300 YES Maximum Displacement 0.000465 0.001800 YES RMS Displacement 0.000156 0.001200 YES
Frequency analysis log file MOS_borazine_freq_new.log
Low frequencies
Low frequencies --- -14.7128 -14.3883 -11.0106 -0.0164 -0.0114 0.0318 Low frequencies --- 289.0449 289.0465 404.2274
Jmol image
optimised borazine molecule |
Comparisons
Charge Distribution
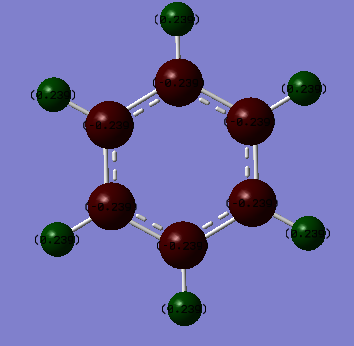
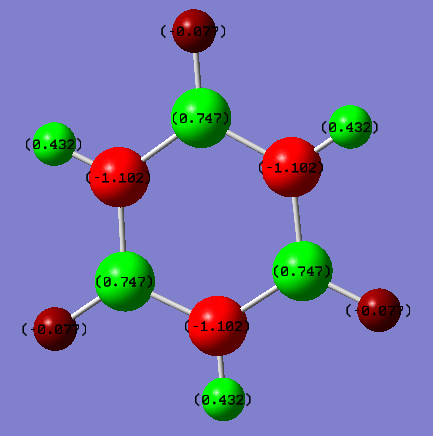
Benzene: The carbon atoms have a charge of -0.084 and the hydrogens have a charge of 0.084. Ng611 (talk) 20:10, 15 May 2018 (BST) That's not what they are in your figure!
Borazine: The boron atoms have a charge of 0.747 and the hydrogens attached have a charge of -0.077. The nitrogen have a charge of -1.102 and the hydrogens attached have a charge of 0.432.
Comparison: An uncharged atom would show as black. This means that the darker the atom, the more neutral the atom. Benzene clearly has much darker colours than borazine so there's a much more neutral spread of charge over the molecule. This makes sense. The ring is made of carbon atoms only with only a single hydrogen atom attached to each. There is no electronegativity difference between each carbon - they're the same atom - so the distribution of charge is only due to the electronegativity difference between the carbons and hydrogens. On the Pauling scale, the electronegativity of carbon is 2.50 and of hydrogen is 2.10. This is not a big difference and is the reason the atoms are much more neutral than in borazine. Nitrogen has an electronegativity of 3.07 and boron of 2.01. This means that the ring itself will have differences in charge and then when you add the hydrogens they will also cause the charges of atoms to change. Boron is also electron deficient so has more of an electron pull to it. It ends up with the molecule you can see - a spread of charges on the atoms, none of them very close to neutral.
- ↑ A complete table of electronegativities" Elbert J. Little Jr. and Mark M. Jones,Journal of Chemical Education, 1960, 37 (5), 231, DOI: 10.1021/ed037p231
Ng611 (talk) 20:07, 15 May 2018 (BST) Good explanation. I would also mention that atoms related by symmetry have identical charges and that the sum of all partial charges is 0.
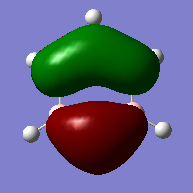
Molecular Orbitals
Concept of Aromaticity
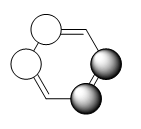
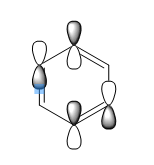
how do the real MOs relate to the common (very basic) conceptions of aromaticity? you are expected to explain why the concept of overlapping pz AOs is NOT a good description for aromaticity.
The common concept of aromaticity - in which the lone electron in each pz orbital, adjacent to the ring, is delocalised over the molecule - is relevant to only one molecular orbital in benzene. It's only a very very small part of the picture of benzene's bonding and aromaticity. Benzene is perhaps the most well known example of an aromatic molecule but it's not the only example by far. Anything that follows Hückel 's rules, so molecules with 4n+2 pi-electrons which are cyclic and planar, can be considered aromatic. Hence, the other molecule that has been studied, borazine, is also an example of an aromatic molecule.
Some of the molecular orbitals in borazine look very similar to orbitals on benzene - with perhaps slightly different shapes due to differences in charge distribution. Others look nothing the same. However, all the molecules in both molecules build up a picture of the bonding and hence aromaticity in the molecules.
The fact that the molecular orbitals aren't all the same, means the common concepts of aromaticity are not very well related to the real MOs. Because if it simply were the case that pz orbitals overlapping caused the aromaticity, the bonding in all aromatic molecules would need to look the same and it doesn't.
The configuration with the p orbitals set up in the way that they could overlap and cause a ring of delocalised electrons is the lowest energy bonding orbital of the MO diagram when looking at just p orbitals in benzene - so it's a very, very simplified version of the picture but not completely abstract - it does make sense.
(sorry, I ran out of time) Ng611 (talk) 20:15, 15 May 2018 (BST) What you;'e written is certainly correct but yes, more detail was needed. In particular, a detailed discussion regarding the modern view of aromaticity influenced by quantum mechanics and other phenomena that influence it. Some discussion regarding how aromaticity can be confirmed experimentally would also be very useful.
Ng611 (talk) 20:15, 15 May 2018 (BST) Generally well laid out and well-written report. A couple of mistakes for the benzene borazine charge analysis but and your discussion of aromaticity needed some more detail. Overall, very well done though!